Can you describe the aim of your grant-winning research in one sentence?
It’s all in the grant title — ‘Establishing a novel human model for anti-fibrotic drug screening in silicosis’.
Sounds like a big and exciting project. Can you tell me a bit about the model — what will it look like, why do we need new models and what are you hoping to see?
Our Discovery Grant from the Dust Diseases Board will be used to investigate silicosis in human precision-cut lung slices (PCLS). This project is so exciting because the PCLS model we are developing contains all the cell types, supportive matrix and structures present in the whole lung. This will allow us to gain unique insights that aren’t possible when studying just one cell type. Our research will help us understand how the human lung responds to both silica and other chemicals that are increased following silica exposure. We hope to show that our novel therapeutics can inhibit lung scarring in PCLS, which will, in turn, support translation to clinical trials for silicosis.
Which anti-fibrotics are you looking at and and why?
We will be testing a range of anti-fibrotic drugs. Currently, the two main options to treat silicosis are whole-lung washout or lung transplant. Washout is highly invasive and may only be of benefit in early disease, while transplants are obviously limited by donor availability.
We will test nintedanib and pirfenidone, currently used for other types of lung fibrosis, and advocate for their use in silicosis. They only slow disease progression, however, so we don’t expect them to provide a cure.
We are also keen to test relaxin, based on our previous work showing that it reverses fibrosis in animal models of lung disease (PMID: 29447958). We’ve further identified increased levels of a cytokine called macrophage migration inhibitory factor (MIF) in silicosis patients, and shown separately that a selective inhibitor of this cytokine reduces silica-induced inflammation in immune cells (PMID: 29884801). If these drugs reduce inflammation and scarring in our PCLS model of silicosis, this will be an important step towards helping patients.
How long before your work affects patient care?
Great question! This will depend on the outcomes from the different therapeutic options we are testing. Repurposing anti-fibrotic therapeutics already approved for clinical use will accelerate their introduction to patient care. The more challenging goal is to validate reversal of silica-induced scarring in our human tissue model. Since this would be a major breakthrough, positive findings would also support a fast-track progression of our novel therapeutics to clinic.
It looks like you’ve done a lot in lung disease research — what have you discovered so far and how will it support this project?
I lead the Respiratory Pharmacology Group within the Biomedicine Discovery Institute at Monash University, and was the first researcher to establish the PCLS technique in Australia. We have performed mechanistic studies and discovered novel therapeutics across multiple lung diseases, mostly in animal models of asthma, COPD, pulmonary hypertension, newborn respiratory failure, lung infections and fibrosis (PMID: 27833558, PMID: 27128803, PMID: 31354700). With support from clinical collaborators, we now have access to human lung tissue. Our development of human PCLS models, including those for silicosis, is really bridging a critical gap to provide mechanistic insights and validation of new treatment targets in lung diseases.
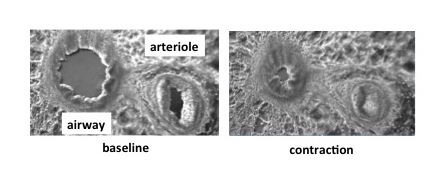
Your Monash profile mentions you developed a technique for visualising changes in intrapulmonary airway and artery lumen area. Briefly, what is the technique, how did it come about and how has it transformed lung disease research?
I was initially trained in the preparation of PCLS by the late Professor Mike Sanderson, an incredibly generous researcher, but have extended its application for studies in disease context. One of the many advantages of this technique is that multiple “precision-cut” slices can be obtained from each lung sample. Each slice contains a cross-section of the smallest airways and arteries that are relatively inaccessible but often the sites of disease. My work has visualised PCLS under phase-contrast microscopy to identify novel dilators as potential treatments for asthma and pulmonary hypertension — it’s amazing to see these drugs working in real-time! (See photo below.) In terms of transforming research, our lab and many others worldwide are now using PCLS to study contraction, inflammation, fibrosis and even responses to infection, all occurring outside the body.