Novel Australian research has confirmed the presence of bacterial biofilm in bronchoalveolar lavage specimens from children with protracted bacterial bronchitis (PBB) or bronchiectasis. Two of the researchers, Dr Ruth Thornton and Dr Robyn Marsh, provide some insight into their study, published in The Lancet Microbe, and its implications.
Can you sum up the aim of this project in 10 words?
To determine biofilm prevalence among kids with chronic wet cough
What aspect of this research excited you the most?
Robyn: We’ve been working with Professor Anne Chang for a while now to understand the microbiology underlying chest infections in kids with PBB or bronchiectasis. Recurrent chest infections are a hallmark of these conditions, even when kids are treated with antibiotics that should work. Being able to partner with Ruth and her team in WA to do this work was terrifically exciting as it meant we could determine whether these kids have biofilm-associated infections. Understanding this is critical to working out how best to treat PBB and prevent bronchiectasis.
Ruth: I have always been excited to unravel the underlying disease pathogenesis in chronic infections that don’t respond well to standard treatments. It is the only way people will start considering alternative treatments which might be more effective. We have done this previously by demonstrating biofilm involvement in otitis media – here and here – and this has led to a clinical trial which is now underway. It would be amazing to be able to help improve treatments for lung disease as well.
What have you previously discovered in the area of biofilms?
Ruth: I previously showed that bacteria persist both in biofilms and intracellularly, in biofilm pods, in the middle ears of children who have chronic and recurrent otitis media. These act as a bacterial reservoir which are not able to be eradicated by antibiotics and these kids end up needing grommet surgery. Robyn and I also previously worked together for a pilot study that demonstrated proof-of-principle that biofilm was present in lung samples from kids with bronchiectasis but we weren’t able to determine biofilm prevalence in this study and didn’t include any kids with PBB.
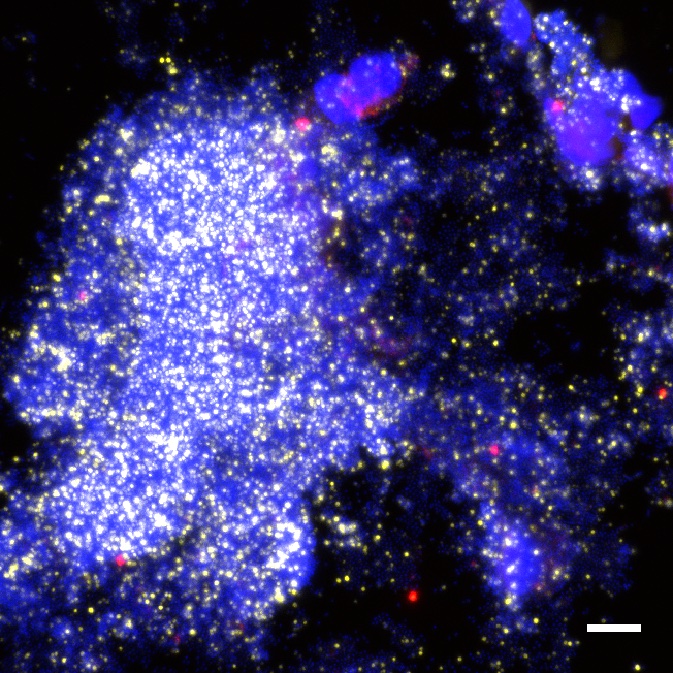
BAL from a child with PBB showing a large polymicrobial biofilm positive for Moraxella catarrhalis
What do we know about how, when and why biofilms form?
Robyn: Bacteria have evolved all sorts of mechanisms that give them a survival advantage in hostile environment. We know from lab-based studies that some bacteria that cause chest infection in kids can produce biofilm, but it’s not a universal response. We don’t understand why biofilm manifests in a subset of children, but it’s likely to be triggered by a range of environmental pressures, including interactions among different bacteria or due to immune pressures. Understanding this is a focus of where our research goes next.
How long before this work might impact on patient care?
Ruth: It is hard to say, but we are hopeful that this won’t be too far away given recent advances in the development of antibiofilm therapies, including treatments that target the host-microbial interactions that are likely essential for biofilm maintenance. We already have a clinical trial for this in otitis media, and there are also promising vaccine candidates that specifically target respiratory biofilms which have the potential to be gamechangers.