While targeted therapies for ALK-rearranged (ALK+) non-small cell lung cancer (NSCLC) have improved outcomes and quality of life for patients, acquired resistance inevitably occurs in patients who initially respond to treatment.1 Molecular profiling at diagnosis and at progression can provide predictors of ongoing therapy response and help individualise patient management.2
The limbic spoke to medical oncologist Doctor Malinda Itchins from the Royal North Shore Hospital about how testing is evolving to provide a better insight into treatment approaches.
Testing for ALK+ NSCLC at diagnosis
“ALK inhibitors are transformative in the treatment of ALK+ NSCLC. Therefore, we need to ensure 100% of NSCLC of predominately adenocarcinoma histology or not-otherwise-specified type are tested at diagnosis for an ALK rearrangement. Unfortunately, given the nature of this unique subtype of lung cancer, many patients present symptomatically or are diagnosed incidentally when they have advanced disease,” explained Dr Itchins. “Challenge number one is to diagnose more patients in general, including those with ALK rearrangements, during early disease so that we can embark upon a curative pathway,” she said.
ALK rearrangements occur in around 3–7% of NSCLCs, and are typically found in adenocarcinomas.1 They occur particularly (although not exclusively) in younger patients who have never smoked, and have a predilection for metastasising to the brain. “In enriched populations such as these, unveiling an ALK rearrangement is not so rare. In Australia in 2021, ALK should be appropriately tested for in all patients with non-squamous NSCLC,” said Dr Itchins.
In Australia, ALK+ NSCLCs are currently detected in tumour specimens using immunohistochemistry (IHC) followed by confirmatory fluorescence in situ hybridization (FISH) in cases of ‘positive’ IHC. These tests identify the presence of ALK rearrangement relatively accurately, but cannot identify the particular ALK fusion partner (also known as the variant). The variant can only be identified via a further test such as reverse transcription polymerase chain reaction (RT-PCR) or next generation sequencing (NGS).1 “NGS is not routinely performed as it is not universally available and rebated in Australia,” explained Dr Itchins.
Repeat biopsies to help guide treatment at progression
Acquired resistance eventually emerges to ALK-directed therapies.1. “At present in Australia for a newly diagnosed patient with an ALK+ NSCLC, we would first use a second-generation ALK inhibitor, such as alectinib or brigatinib – both PBS reimbursed – then at progression we would empirically opt for the third generation ALK inhibitor, lorlatinib. We also always think about enrolling the patient into a clinical trial, if available.” explained Dr Itchins. “Lorlatinib was developed to be active post these earlier generation agents, particularly for a common cause of resistance: the development of a pan-resistant ALK G1202R mutation. A proportion of patients, however, will not respond to lorlatinib, or may even respond to an earlier generation ALK inhibitor sequenced in at this point, for example brigatinib, post-alectinib. Unfortunately, in the absence of re-biopsy via a tissue or plasma sample and NGS, we cannot be confident in selecting this approach as the ALK mutations conferring resistance to alectinib may also result in brigatinib being ineffective. In current practice, we are treating ‘blindly’ and hoping we have made the right choice, based on probabilities from small trial datasets,” she explained. “For many patients, they need a clinical response and may not have time for trial and error. Biomarker testing, enabled by NGS, may guide drug selection and promote best outcomes, including beyond first and second lines of therapy, where we have the most data,” she said.
Table: Cellular ALK Phosphorylation Mean IC50 (nM) in Ba/F3 cells harbouring wild-type EML4-ALK variant 1 (V1) and EML4-ALK resistance mutants
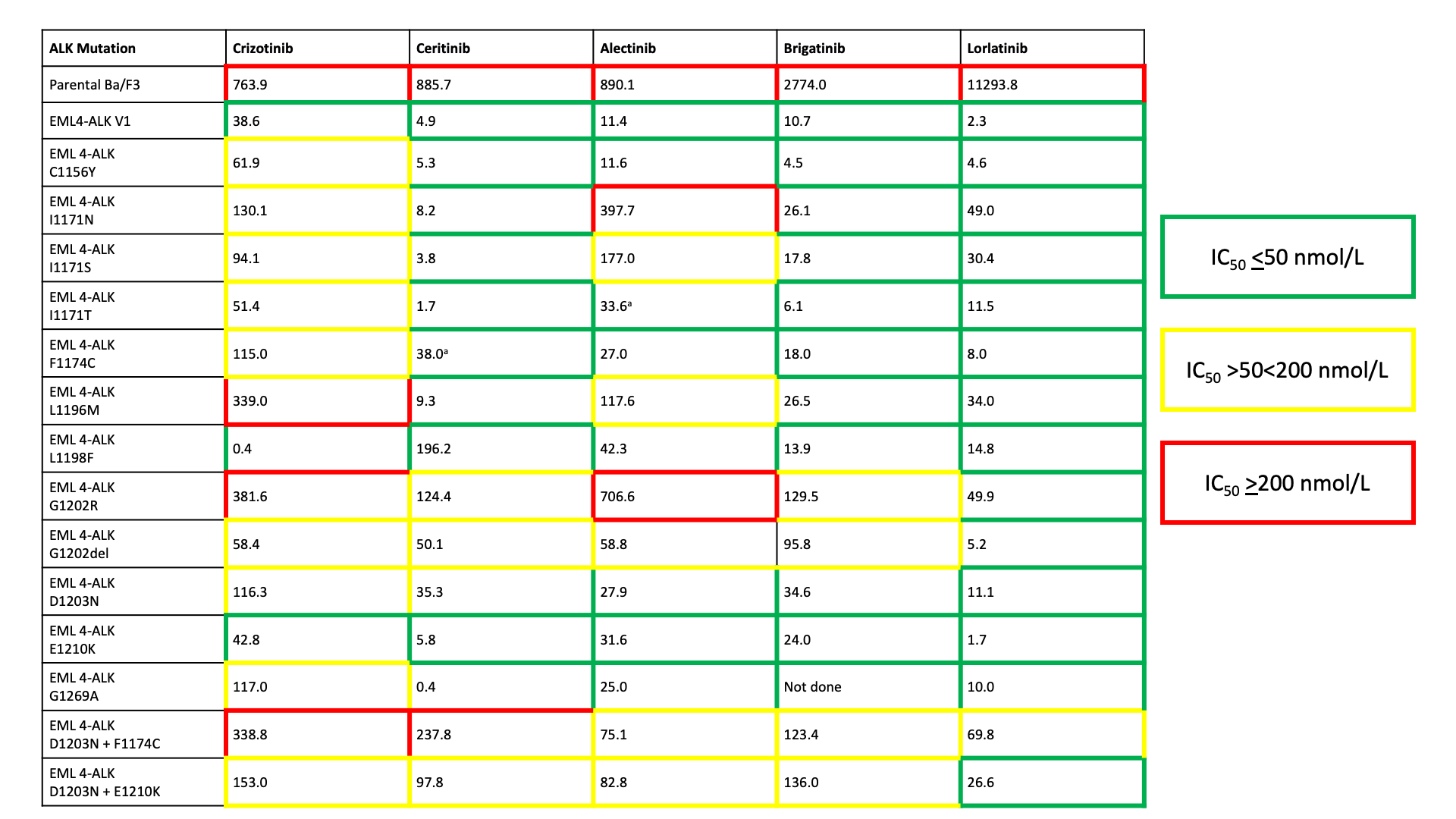
aThese mutations may not be susceptible to the agent in vivo based upon previous clinical reports, but appeared sensitive in Ba/F3 cells. Adapted from Gainor et al 20163
At disease progression, not only can genomic profiling (if available) be very useful to identify the mechanism of acquired resistance via the emergence of any ALK mutation(s) conferring sensitivity to an alternate ALK inhibitor, but also test the emergence of an ALK independent resistance pathway, such as bypass tract variants (e.g. MET amplification). “This critical information can help us decide on the next line of therapy in a non-empiric fashion and with greater confidence,” explained Dr Itchins.
“Another important reason to re-biopsy patients at progression is because the biology of the disease can transform, especially with the use of later-generation more potent ALK inhibitors,” said Dr Itchins. “I like to see the repeat ALK IHC result to make sure we are still likely dealing with an ALK-driven cancer,” she explained. “I’ve had examples in my practice of small cell and squamous transformation, which importantly directs a different systemic treatment (and prognostic) pathway,” she said.
“Testing at resistance, of course, needs to be done on an updated tissue biopsy, as a spectrum of different ALK mutations can emerge following different ALK inhibitors, and these mutations further vary in responsiveness to different ALK inhibitors, according to current literature,”3 said Dr Itchins. “Some locally available ‘hot spot’ molecular panels can give a limited array of ALK resistance mutations and may help us understand why an ALK inhibitor is not working and help with the next choice of therapy. When treating molecularly-driven lung cancers, as with all cancers, we navigate the current system as best we can to access all of the biological information possible and deliver optimal care at each step of a patient’s lung cancer journey,” she said. “I should note it is important to biopsy a growing lesion at resistance, and an FDG-PET may help inform best site selection. A tissue biopsy will not capture the potential spatial heterogeneity at play in a case of drug resistance, an area where liquid biopsy is proving increasingly valuable, complementary and non-invasive.”
“In Australia, we currently do not have universal access to the depth of genomic sequencing we would like for lung cancer, particularly to meet best standards of personalised medicine in treating ALK, or for diagnosing and managing an array of other oncogenes in lung cancer. Complex genomic profiling (CGP) is not currently funded by Medicare, so an individual would need to pay for such a panel, or the clinician needs to strategically access the assay through a clinical trial or research partnership. Some centres may have ‘hotspot’ NGS panels available and reimbursed for our reflex EGFR testing, and these panels may encompass part of the ALK kinase domain, so some of the canonical ALK resistance mutations may be captured. This access across Australia is patchy and, as you can see, there is no standard practice. Testing is on a per-patient basis, when the treating doctor actively seeks more information, which is also not standardised in this evolving area,” explained Dr Itchins.
The future role of NGS in guiding therapy
In the future, the wider availability of NGS may play a role in diagnosis, monitoring, drug resistance profiling, and helping to guide a more personalised treatment approach.2 NGS will also help provide further understanding of the biology of ALK+ NSCLC.2
While not yet routinely available in Australia, this technology is being employed in the clinical trial setting, which provides a potential avenue to access for eligible patients.
One clinical trial using such technology is ASPiRATION. This is a multicenter prospective observational cohort study currently underway to assess the impact and feasibility of Comprehensive Genomic Profiling (CGP) in generating genomic information to personalise clinical decision making. The study is a collaboration between the Thoracic Oncology Group of Australasia (TOGA), the Australian Genomic Cancer Medicine Centre, and the NHMRC Clinical Trials Centre, University of Sydney.
Patients eligible for the trial include adults with newly diagnosed, pathologically-confirmed metastatic nonsquamous NSCLC and sufficient and accessible tissue for molecular screening. This trial will also detect ALK+ NSCLC, ensuring that all patients with an ALK rearrangement are detected, including those with a potentially false-negative IHC and/or FISH test.
Clinicians can find out more about the ASPiRATION trial here. There is at least one site open in each state and territory.
“NGS also identifies the particular ALK variant, which is emerging as a guide in selecting the appropriate ALK inhibitor. We are learning more about the susceptibility and the unique resistance profiles of different variants,” explained Dr Itchins.
“For example, in V1, brigatinib and alectinib are very active; however, for V3, lorlatinib may be a superior upfront choice (according to emerging evidence in the absence of randomisation). Lorlatinib is currently not indicated for upfront treatment, and we’re waiting on the mature front-line lorlatinib CROWN data and PBAC review to determine whether the decision to use lorlatinib upfront will be something we’ll be grappling with here in Australia in the future,” said Dr Itchins. “This is a good problem to be having.”
Expanded panels may also detect the presence of co-mutations – a common example being TP53 variants, where evidence suggests there’s an attenuated PFS to ALK-inhibitor therapy and worse prognosis. “Having such information to hand from the outset will help us define a group of patients who may need more than monotherapy and who would benefit from enrolment into an appropriate clinical trial,” said Dr Itchins.
‘Best first’ strategy for sequencing ALK inhibitors
Although genetic resistance testing is, to an extent, currently playing a role and likely to play a larger future role in guiding the choice of therapy, the treatment algorithm is currently informed by efficacy data from clinical trials in a non-biomarker guided setting.
The second-generation ALK inhibitors brigatinib and alectinib are now established first-line treatments for patients with ALK+ NSCLC due to their higher systemic and cranial efficacy compared to first-generation crizotinib which has now been replaced.4,5 Brigatinib is also a preferred second-line treatment for patients progressing on crizotinib, based on the results of clinical trials showing its impressive efficacy in crizotinib-refractory disease.2,3 Third-generation lorlatinib is available for progression after second-generation ALK inhibitors.2
“You always want to use the most potent ALK inhibitor upfront,” said Dr Itchins. “We can’t guarantee that the patient will be well enough to receive a next line of therapy at relapse, so we can’t hold something back that we may never use,” she explained. “We need to be hopeful that our patients perform well but always be prepared for things not behaving as we would like. In the future, the use of temporal tissue and plasma genetic sequencing to guide selection of therapies in an informed personalised manner – based on a more comprehensive genomic signature – I am certain will take us to new heights in treating this disease” she said.
Disclosure
This article was sponsored by Takeda Pharmaceuticals. Any views expressed in the article are those of the expert alone and do not necessarily reflect the views of the sponsor. Before prescribing, please review the ALUNBRIG (brigatinib) product information via the TGA website. Treatment decisions based on these data are the responsibility of the prescribing physician.
References
- Itchins M et al. Treatment of ALK-rearranged non-small cell lung cancer: a review of the landscape and approach to emerging patterns of treatment resistance in the Australian context. Asia-Pac J Clin Oncol 2017;13:3–13. https://pubmed.ncbi.nlm.nih.gov/28795492/
- Elsayed M and Christopoulos P. Therapeutic sequencing in ALK+ NSCLC. Pharmaceuticals 2021;14:80. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7912146/
- Gainor JF, Dardaei L, Yoda S, Friboulet L, Leshchiner I, Katayama R, Dagogo-Jack I, Gadgeel S, Schultz K, Singh M, Chin E. Molecular mechanisms of resistance to first-and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer discovery. 2016 Oct 1;6(10):1118-33.
- Huber RM et al. Brigatinib in crizotinib-refractory ALK+ NSCLC: 2-year follow-up on systemic and intracranial outcomes in the phase 2 ALTA trial. JTO 2019;15(3):404–415. https://pubmed.ncbi.nlm.nih.gov/31756496/
- Mok T. Survival and final progression-free survival data for treatment-naïve advanced ALK-positive non-small-cell lung cancer in the ALEX study. Annals of Oncology 2020; 31(8):1056–1064. https://www.annalsofoncology.org/article/S0923-7534(20)39796-9/fulltext
