The TGA is alerting clinicians to a counterfeit version of the American approved product carmustine for injection (BiCNU) 100mg following its discovery outside the US.
In an alert issued this week the TGA says it is unlikely that the counterfeit product will enter the Australian market because the counterfeit product has the US label and a grey coloured flip top. The Australian product has a blue flip top.
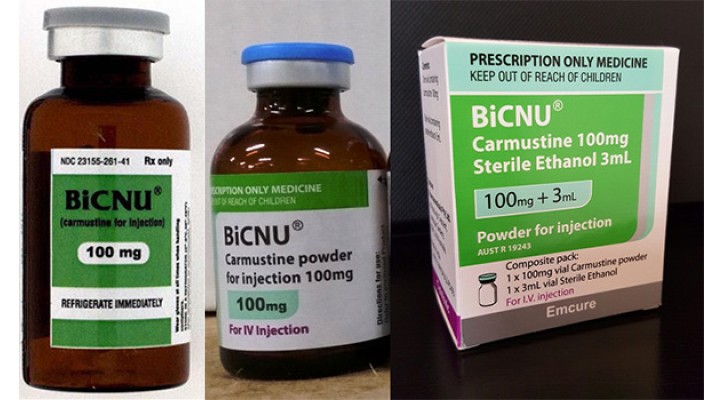
Conterfeit version on the left. The Australian product on the right has a number of differences
The US approved product states ‘Rx Only’ where the Australian approved product (bottle and outer box) states PRESCRIPTION ONLY MEDICINE KEEP OUT OF REACH OF CHILDREN. The Australian approved product (bottle and outer box) contains the unique ARTG Registration Number AUSTR 19243.
BiCNU containing carmustine is indicated as palliative therapy as a single agent or in established combination therapy with other approved chemotherapeutic agents in the following:
- Malignant Glioma.
- Multiple Myeloma: In combination with prednisone.
- Hodgkin’s Disease: As secondary therapy in combination with other approved drugs in patients who relapse while being treated with primary therapy, or who fail to respond to primary therapy.
- Non-Hodgkins lymphomas: As secondary therapy in combination with other approved drugs for patients who relapse while being treated with primary therapy or who fail to respond to primary therapy.
In the unlikely event that a counterfeit product is discovered the TGA should be contacted immediately.