Faecal microbiota transplantation (FMT) may produce short-term improvements in symptoms in patients with irritable bowel syndrome but the benefits are not maintained at one year, a randomised controlled trial in 65 patients has shown.
However, another RCT presented at DDW 2018 found no improvement in patients who had FMT compared to placebo.
A Belgian trial of nasogastric or duodenal delivery of FMT from two healthy donors showed there were significant improvements in the primary outcome of IBS symptoms at 12 weeks, compared to placebo (patient’s own frozen stool).
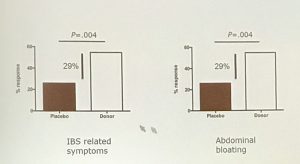
The double blind trial showed that at 12 weeks the 43 IBS patients who received FMT had significantly better response rates in self reported IBS symptoms such as abdominal bloating (59% vs 29%) compared to 19 patients who received placebo.
 The FMT group also showed significant improvements in the secondary outcomes of IBS-related quality of life and other IBS symptoms such as pain and urgency, according to study investigator Dr Tom Holvoet, of the Department of Gastroenterology, University Hospital Ghent.
The FMT group also showed significant improvements in the secondary outcomes of IBS-related quality of life and other IBS symptoms such as pain and urgency, according to study investigator Dr Tom Holvoet, of the Department of Gastroenterology, University Hospital Ghent.
However only six of 22 responders (27%) followed up at 12 months showed a sustained response, while 16 (73%) had relapsed, he noted.
Analysis of the microbiota showed a significant difference in species proficle between unsuccessful placebo recipients and successful FMT recipients.
“FMT induces significant changes to the microbiota composition and these appear to be related to treatment success,” said Dr Holvoet.
“Non-responders [are] more similar to placebo patients than to successfully treated patients,” he added.
Dr Holvoet said most of the experience of FMT in IBS had come from case reports or pilot studies, and this trial was one of the first larger scale double blind RCTs.
Presenting the findings at Digestive Disease Week 2018 in Washington DC, he said the results suggested that FMT could be a successful treatment in IBS, but with only moderate long term effects.
The trial results highlighted unanswered questions on the effect of patient selection and frequency of treatment with FMT, he said.
Further research was needed on the effect of the form of facecal transplant used – such as frozen capsules, and the number of source donors – on the efficacy in IBS, he added.
Meanwhile, preliminary results of a Danish double blind trial of FMT capsules in 52 adult patients with moderate to severe IBS showed no benefit of treatment.
Patients were randomised to FMT or placebo capsules for 12 days and followed for six months. While patients who received FMT capsules had an increase in biodiversity to become similar to the donors, no significant difference in improvement in IBS-SSS score was observed 3 months after treatment. In fact, patients in the placebo-group experienced greater symptom relief and quality of life compared to the FMT group.
“Altering the gut microbiota is not enough to obtain clinical improvement in IBS,” the authors concluded.