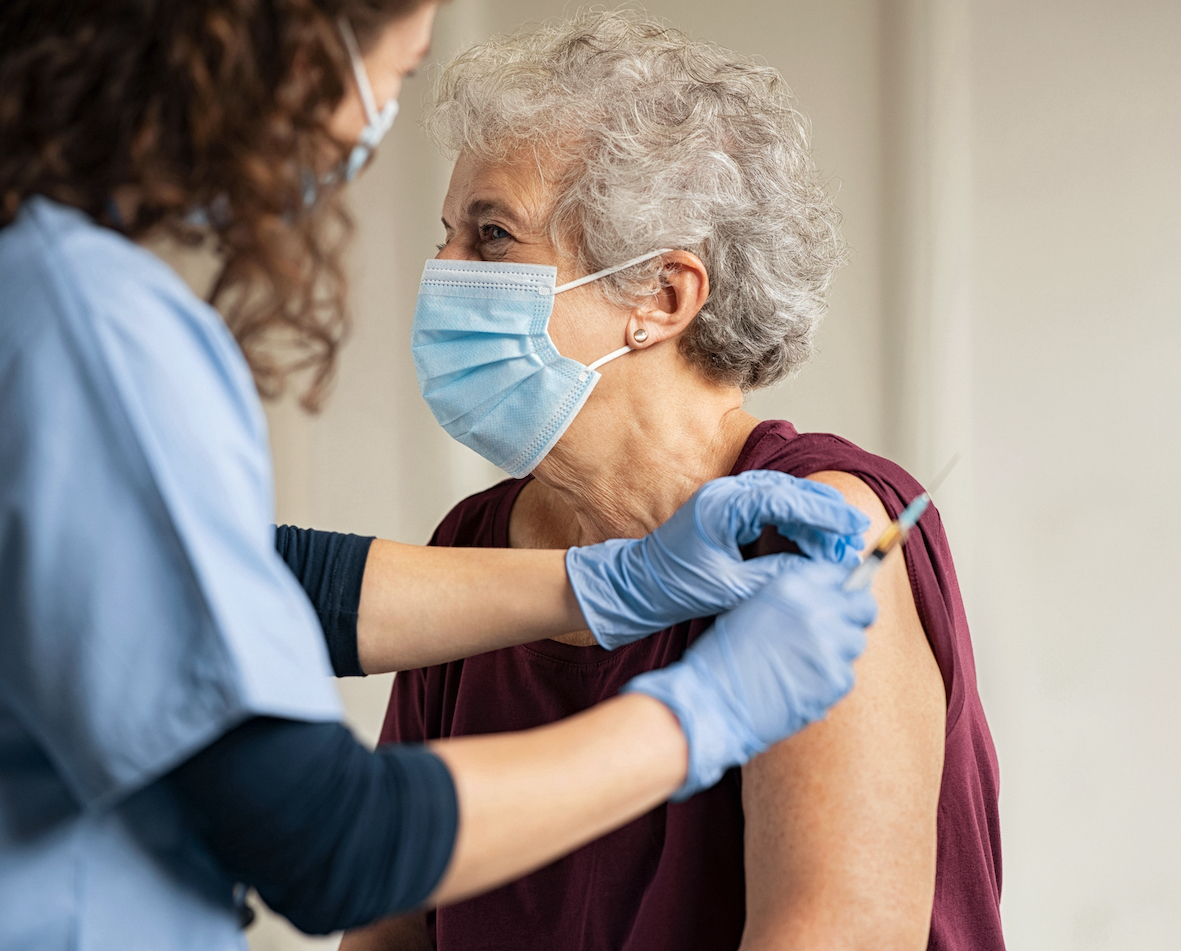
 The Australian Technical Advisory Group on Immunisation (ATAGI) have updated their recommendations related to the new COVID-19 vaccination related thrombotic syndrome predominantly seen in Europe and the UK and recently in an Australian case.
The Australian Technical Advisory Group on Immunisation (ATAGI) have updated their recommendations related to the new COVID-19 vaccination related thrombotic syndrome predominantly seen in Europe and the UK and recently in an Australian case.
The new recommendations are that:
1. The use of the Pfizer vaccine is preferred over the AstraZeneca vaccine in adults aged less than 50 years who have not already received a first dose of AstraZeneca vaccine. This is based both on the increased risk of complications from COVID-19 with increasing age, and thus increased benefit of the vaccination, and the potentially lower, but not zero risk, of this rare event with increasing age.
2. Immunisation providers should only give a first dose of AstraZeneca COVID-19 vaccine to adults under 50 years of age where benefit clearly outweighs the risk for that individual’s circumstances
3. People who have had their first dose of the COVID-19 AstraZeneca without any serious adverse events can safely be given their second dose. This includes adults under the age of 50. People who have had blood clots associated with low platelet levels after their first dose of COVID-19 AstraZeneca should not be given the second dose.
4. The Department of Health further develop and refine resources for informed consent that clearly convey the benefits and the risks of the AstraZeneca vaccine for both immunisation providers and consumers of all ages.
Recently, the Thrombosis and Haemostasis Society of Australia and New Zealand (THANZ) issued an advisory statement on vaccine induced prothrombotic immune thrombocytopenia (VIPIT).
As reported recently in the limbic, anti-platelet antibodies appear to be associated with rare but severe COVID-19 vaccination-related thrombotic complications similar to heparin induced thrombocytopenia (HIT).
The THANZ advisory statement said understanding of the new syndrome was rapidly evolving.
What to look for
It said most reported cases of VIPIT have presented with cerebral venous sinus thrombosis but any patients with symptoms of thrombosis shortly after vaccination should be considered carefully.
“We suspect the timing of greatest risk is between days 4-20 based on case reports to date,” the statement by the THANZ vaccine thrombocytopenia working group said.
Other features of cases to date include:
- Thrombocytopenia
- High, typically very high D-dimer
- Some patients refractory to standard anticoagulation
- Some response to IVIG
Testing