An international team of microbiologists have made a breakthrough in understanding how the superbug Pseudomonas aeruginosa causes infection.
Associate Professor Cynthia Whitchurch, a microbiologist from UTS Science’s ithree Institute and colleagues found that Pseudomonas aeruginosa cells explode violently, a finding that they say could hold the key to explaining how the superbug can cause infections with potentially deadly consequences.
“When most people think about bacterial cell death, they think of the cell dying and their contents slowly leaking out, similar to what you would see with a piece of fruit rotting,” said Professor Whitchurch.
“What’s so amazing about this discovery is that we now know the bacteria have a process that enables them to actively explode, and therefore efficiently release all of their internal contents, making these available for use by the remaining members of their community.”
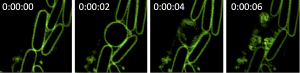
Co-author Dr Lynne Turnbull who is also from the UTS said the Pseudomonas cells undergo an incredible transformation before exploding with the whole process, in some instances, taking place in just six seconds.
“The normal bacteria look like little rods or pills,” she said. “One day, as we looked under the microscope, we saw one of the cells turn from a hard, structured rod into a round, soft ball. Within a few more seconds, it then violently exploded—it’s amazing how quickly it happens and is likely the reason it hasn’t been observed before.”
The team identified a gene specifically involved in the process of Pseudomonas aeruginosa cells rounding up and exploding.
Associate Professor Whitchurch said that the mechanism was surprising and added another element to their thinking about the role of bacteriophage in bacterial populations.
“You wouldn’t think that having a continuous viral infection was a good thing but the viral DNA in the genome of the bacteria is providing an advantage to the population – it’s being maintained for a reason.”
Thee researchers used a conventional microscope for their “fireworks” findings but used a more high-tech microscope* to see what happens after the bacterium exploded.
“The super-resolution microscopy technology was a very important part of solving the puzzle,” Associate Professor Whitchurch said.
The researchers will now turn their attention to understanding the specific role of exploding cells in infection. They predict it will be important not only in biofilm infections, but also in tackling the global problem of antibiotic resistance.
“We think there will be a two pronged approach: one is to prevent the biofilms being produced by stopping bacterial explosions, but if we can’t prevent it, then the second approach will be to induce the process that cause the bacteria to explode, so they all die en masse causing the infection to clear,” Associate Professor Whitchurch said.
“We know we can induce this explosive cell death pathway through antibiotic treatment, so maybe we can use this to kill whole populations of bacteria—it’s a potential therapy.
“We know certain classes of antibiotics can induce this pathway, however now that we know about how cells explode, we have an opportunity to directly target that process more specifically, perhaps with new types of antibiotics, or by finding drugs or chemicals that cause this process to be turned on.”
*The DeltaVision OMX Blaze™ is manufactured by Applied Precision, a GE Healthcare company based in the USA.
This research paper ‘Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms’ was published in Nature Communications and can be viewed at: 10.1038/NCOMMS11220
Adapted from a press release published by the UTS.