The US Food and Drug Administration (FDA) has added a boxed warning to febuxostat after a postmarketing safety clinical trial found an increased risk of cardiovascular-related death and all-cause mortality compared to allopurinol.
The FDA said it was also limiting the approved use of febuxostat to second-line treatment in gout after allopurinol.
“Health care professionals should reserve [febuxostat] for use only in patients who have failed or do not tolerate allopurinol,” it said in a statement.
Patients with cardiovascular risk should be advised of potential cardiovascular symptoms and counselled to seek medical help immediately if they experience them, the FDA advised.
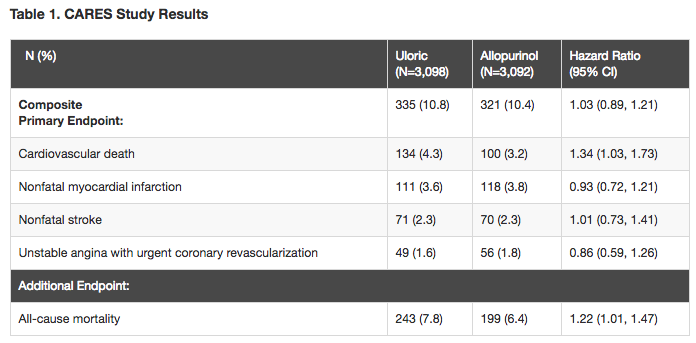
The FDA said its decision was based on data from the Cardiovascular Safety of Febuxostat and Allopurinol in Patients with Gout and Cardiovascular Morbidities (CARES) study, a multicentre, randomised, double-blind cardiovascular outcomes trial conducted in 6,190 patients with gout treated with either febuxostat or allopurinol (see table below).
The results showed that although the study met the pre-specified noninferiority margin, there was a significant increase in cardiovascular death. In addition, there was a significant increase in overall mortality, which was driven by cardiovascular death.
“In patients treated with [febuxostat], 15 deaths from heart-related causes were observed for every 1,000 patients treated for a year compared to 11 deaths from heart-related causes per 1,000 patients treated with allopurinol for a year.
“In addition, there were 26 deaths from any cause per 1,000 patients treated for a year with [febuxostat] compared to 22 deaths per 1,000 patients treated for a year with allopurinol.
The TGA’s product information for febuxostat includes an advisory statement that treatment with the drug is not recommended in patients with ischaemic heart disease or congestive heart failure.
A spokesperson for the Therapeutic Goods Administration (TGA) told the limbic: “The TGA is aware of the FDA decision to limit the use of febuxostat (marketed in Australia as Adenuric) to a second-line treatment for gout due an increase in heart-related deaths shown in a clinical trial. The TGA has been in contact with the Australian sponsor and is investigating this issue to determine whether any regulatory action is required in the local context.
“The PBS-listing already restricts febuxostat to second line treatment of gout, which is consistent with the recent FDA changes.”
The manufacturer, Menarini said it “is aware of the FDA decision and [is] liaising with the TGA on appropriate next steps in Australia”.