In a recent Amgen-sponsored Multiple Myeloma Expert Forum in July 2021, Professor Paul Richardson from Harvard Medical School described the updates to the EHA-ESMO Clinical Practice Guidelines for patients with relapsed/refractory multiple myeloma (RRMM).
Patients who have had one prior line of therapy
Figure 1 shows the updated Guidelines algorithm for second-line options in patients with multiple myeloma who were treated with VRd or daratumumab-based (Dara-based) therapies in the first-line setting.1
Figure 1. Recommendations for second-line options for MM patients following VRd and Dara-based first-line therapies1
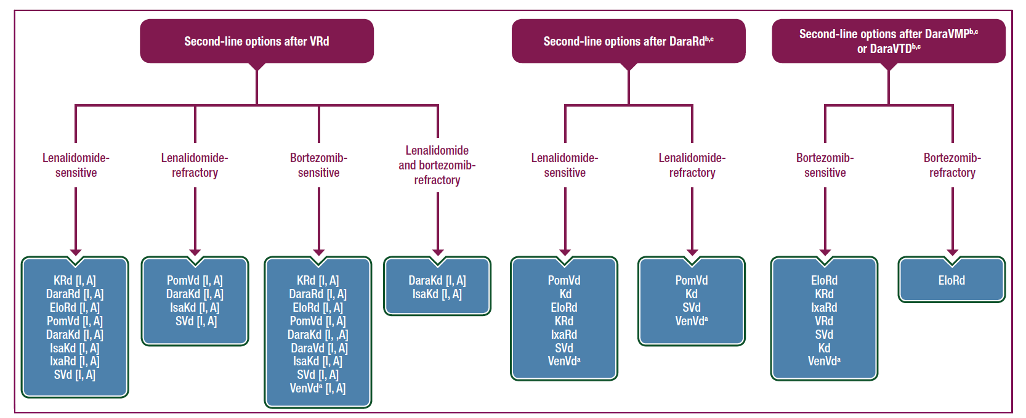
aPatients with t(11;14); bPatients who progress while on monthly Dara are considered as Dara-refractory; cAll recommendations for patients who receive front-line therapy with Dara-based therapies are based on panel consensus as there are no trials evaluating regimens in second-line therapy that include patients refractory or exposed to Dara.
The American and European Associations for Bone Marrow Transplantation developed the recommendation that salvage ASCT be considered for any patient relapsing after primary therapy who achieved at least 18 months remission following their initial transplant.1,2 However, this recommendation was made prior to the introduction of lenalidomide as routine maintenance therapy. The updated EHA-ESMO Clinical Practice Guidelines included the panel consensus that for patients who have had lenalidomide maintenance therapy, salvage ASCT may still be relevant, with opinion suggesting consideration in patients with an initial remission duration of 36 months.1
What about re-induction prior to salvage ASCT? The updated guidelines highlighted that only retrospective data exists, providing the basis of the recommendation that it may not offer a survival benefit the second time around.1
Where salvage is not possible, a lenalidomide-based second-line therapy is recommended in combination with a proteosome inhibitor (PI) or monoclonal antibody (mAb) combined with dexamethasone to offer a progression-free survival (PFS) benefit over lenalidomide-dexamethasone alone.1
Patients in whom lenalidomide is no longer an appropriate treatment option could receive either Kd or DaraVd.1 However, it’s worth noting that the upfront use of Dara-based regimens poses challenges in the second and subsequent lines of therapy as there is no evidence for daratumumab retreatment in the second line setting.1
Two or more lines of prior therapy
For patients who have received two or more lines of therapy, treatment is becoming a challenge. An analysis looking at outcomes in patients with MM refractory to anti-CD38 mAbs found that the median overall survival (OS) for patients refractory to two PIs, two immunomodulatory drugs (IMiDs) and an anti-CD38 mAb was 5.6 months (n=70, 95% CI 3.5, 7.8).1,3
The EHA-ESMO guidelines recommend considering DaraKd or IsaKd for patients who are refractory to bortezomib and lenalidomide, who have not received a mAb. For subsequent lines of therapy, the addition of pomalidomide may be an option.1 Figure 2 outlines the recommended options at second or subsequent relapse.
Figure 2. Recommendations for second or subsequent relapse of MM1
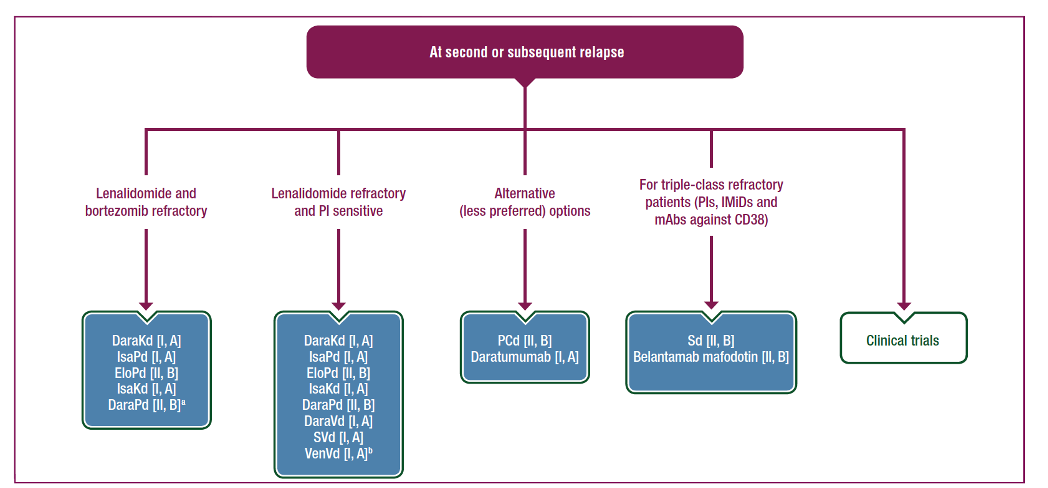
aOnly phase IB data are published for DaraPd. Publication of phase III data are expected in 2021. bFor patients with t(11;14) or high BCL2 levels.
Patients with high-risk characteristics
Patients with the t(11;14) chromosome translocation who are PI-sensitive may derive benefit from B-cell lymphoma (Bcl)-2 inhibitor therapy. This is based on data from the phase III BELLINI trial which looked at a Bcl-2 inhibitor (venetoclax) in combination with bortezomib and dexamethasone (VenVd) vs Vd alone in RRMM patients who had received 1-3 prior lines of therapy, and who were PI-sensitive. In patients with t(11:14), VenVd showed a significant PFS benefit (HR 0.10; p=0.003).1,4 The EHA-ESMO guidelines therefore recommend VenVd as a suitable option for patients with t(11:14) who have received 1 to 3 prior lines of therapy and are PI-sensitive.1
In closing, Prof. Richardson noted how innovations to date produced significant improvements in PFS and OS and the next wave of innovations will involve both novel therapies as well as novel combinations.
ASCT=autologous stem cell transplantation; CI=confidence interval; DaraKd=daratumumab, carfilzomib, dexamethasone; DaraPd=daratumumab, pomalidomide, dexamethasone; DaraRd=daratumumab, lenalidomide, dexamethasone; DaraVd=daratumumab, bortezomib, dexamethasone; DaraVMP= daratumumab, bortezomib, melphalan, prednisone; DaraVTd=daratumumab, bortezomib, thalidomide, dexamethasone; EHA-ESMO=European Hematology Association and European Society for Medical Oncology; EloPd=elotuzumab, pomalidomide, dexamethasone; EloRd=elotuzumab, lenalidomide, dexamethasone; IsaKd=isatuximab, carfilzomib, dexamethsone; IsaPd=isatuximab, pomalidomide, dexamethasone; IxaRd=ixazomib, lenalidomide, dexamethasone; Kd=carfilzomib, dexamethasone; KRd=carfilzomib, lenalidomide, dexamethasone; MM=multiple myeloma; PCd=pomalidomide, cyclophosphamide, dexamethasone; PomVd=pomalidomide, bortezomib, dexamethasone; Sd=selinexor, dexamethasone; SVd=selinexor, bortezomib, dexamethasone; VenVd=venetoclax, bortezomib, dexamethasone; VRd=bortezomib, lenalidomide, dexamethasone;
References:
- Dimopoulos MA, et al. Ann Oncol 2021;32(3):309-322.
- Giralt S, et al. Biol Blood Marrow Transplant. 2015;21:2039-2051.
- Gandhi UH, et al. Leukemia. 2019;33:2266-2275.
- Harrison S, et al. Blood. 2019;134:142.