In a recent Amgen-sponsored Multiple Myeloma Expert Forum in July 2021, Professor Paul Richardson from Harvard Medical School described frontline patient management concepts presented this year at the EHA2021 Virtual Congress. He said the talk of the town was ‘don’t save the best till last’ and ‘one size does not fit all’ in treating newly diagnosed multiple myeloma (NDMM). While not all regimens discussed are available in Australia due to registration and reimbursement status, the predicted trend in NDMM is towards earlier, more aggressive therapy.
Standard of care for fit, newly diagnosed patients aged <70 years:
Figure 1 shows the updated EHA-ESMO Clinical Practice Guidelines algorithm for induction, consolidation and maintenance treatment for multiple myeloma in fit, newly diagnosed patients under the age of 70 years.1
Figure 1. Recommendations for first-line therapy in NDMM patients1
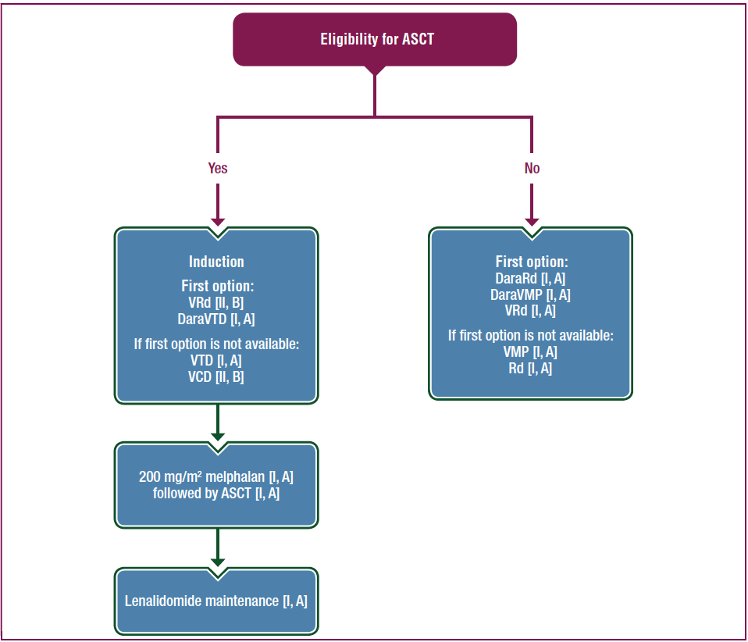
ASCT=autologous stem cell transplantation; DaraRd=daratumumab, lenalidomide, dexamethasone; DaraVMP= daratumumab, bortezomib, melphalan, prednisone; DaraVTD=daratumumab, bortezomib, thalidomide, dexamethasone; Rd=lenalidomide, dexamethasone; VCD=bortezomib, cyclophosphamide, dexamethasone; VMP=bortezomib, melphalan, prednisone; VRd=bortezomib, lenalidomide, dexamethasone; VTD=bortezomib, thalidomide, dexamethasone.
Induction regimen
The EHA-ESMO guidelines suggest a triplet induction regimen with a bortezomib/dexamethasone backbone followed by ASCT as the current standard of care for fit, newly diagnosed patients.1 Guidance on the use of a specific proteasome inhibitor (PI) backbone still lacks definitive head-to-head trial data, although data from the elderly cohort of the ENDURANCE trial showed there is no progression-free survival (PFS) difference between KRd and VRd regimens in transplant-eligible patients.1
A PI plus an immunomodulatory drug (IMiD) is preferred over chemotherapy, with VTd showing better response rates over VCd. PAd was as effective as VCd, but VCd was more tolerable.1
There is also growing interest in quadruplet regimens given the data emerging from the CASSIOPEIA trial. This phase III trial compared 4 cycles of induction with VTd (n=542) with 4 cycles of daratumumab plus VTd (DaraVTd; n=543) before patients received a single ASCT, followed by consolidation and maintenance. In this trial the PFS at 18 months showed DaraVTd was superior to VTd, 93% versus 85% (p<0.0001).1,2 The PFS advantage of quadruplet therapy over triplet therapy was also apparent in the phase II GRIFFIN study, comparing daratumumab plus VRd (DaraVRd) to VRd alone. This study showed 24-month PFS rates of 96% for DaraVRd versus 90% for VRd (p value not shown).1.3
Consolidation and maintenance therapy
Consolidation therapy post ASCT has not been established as a standard of care. In patients who have received VCd, 2 cycles of VRd consolidation may be considered, while tandem ASCT may be considered for genetically-defined high-risk disease.1
Following ASCT, maintenance therapy with lenalidomide is still recommended. This comes from the more than 2-year PFS and 2.5-year overall survival (OS) benefit of lenalidomide over placebo shown in a meta-analysis of 1,200 patients followed up to 79.5 months.1,4 In Europe and the US, lenalidomide maintenance therapy is used post-ASCT for all patients until disease progression.1,5
What about transplant-ineligible patients?
Prior to 2019, VMP and lenalidomide plus dexamethasone (Rd) were the standards of care. However, a phase III trial of 525 NDMM patients demonstrated that VRd is superior to Rd in terms of PFS and OS (median PFS 41 versus 29 months, p=0.003; median OS not reached versus 69 months, p=0.0114).1 This led to the updated recommendation to include VRd as a standard of care in patients not eligible for ASCT. Substitution of bortezomib with carfilzomib offers similar results.1
The addition of daratumumab to VMP and Rd regimens has led to the creation of two new standards of care.1 This was based on data from two phase III studies, ALCYONE and MAIA.1,6,7 ALCYONE describes the impact of DaraVMP followed by daratumumab given until disease progression and showed an improvement in PFS and OS at 40 months (median PFS: 36.4 versus 19.3 months for DaraVMP and VMP, respectively; overall survival rate at 36 months was 78% versus 68% for DaraVMP and VMP respectively, HR 0.60, p=0.0003).1,6
The MAIA study showed the same for daratumumab plus Rd (DaraRd) followed by daratumumab at 30 months follow-up (PFS 70.6% versus 55.6% for DaraRd and Rd, respectively, HR 0.56, p<0.001).1,7
What is the role of transplant?
Does transplant confer the benefit we expect in the long term? A recent paper by Devarakonda et al. (2021) discussed this question8 that appeared to be on peoples’ minds at EHA 2021.
If we turn the clock back a few decades, alkylating agents and corticosteroids were the standard of care, yet did little to move the needle on disease outcome. High-dose melphalan was introduced to help ameliorate drug resistance yet came at the cost of prolonged myelosuppression.8 Then came ASCT, which aimed to repopulate the stem cell population, with greater success than conventional chemptherapy.8 Yet, while ASCT has been shown to improve response rate and PFS, most trials have failed to detect a significant benefit in OS. Furthermore, there is no consensus regarding the ideal pre-transplant response to chemotherapy that dictates the ideal implementation of ASCT nor timing of the transplant.8
Unlike chemotherapy, targeted agents such as the IMiDs and PIs affect not only tumour cells, but it is thought they interact with the tumour microenvironment as well.8 Therefore the pitfalls of chemotherapy may not be the same for these newer agents.8
Several trials are ongoing to determine whether minimal residual disease (MRD) testing could be a future biomarker for timing of ASCT.8 We know that MRD negativity is associated with longer PFS and OS and, if sustained, is the first step towards functional cure for MM.8
In concluding, Prof. Richardson noted that when it comes to timing of ASCT in the era of novel agents, one size does not fit all.
ASCT=autologous stem cell transplantation; DaraRd=daratumumab, lenalidomide, dexamethasone; DaraVMP= daratumumab, bortezomib, melphalan, prednisone; DaraVTd=daratumumab, bortezomib, thalidomide, dexamethasone; KRd= carfilzomib, lenalidomide, dexamethasone; MM=multiple myeloma; PAd=bortezomib, doxorubicin, dexamethasone; Rd=lenalidomide, dexamethasone; VCd=bortezomib, cyclophosphamide, dexamethasone; VMP=bortezomib, melphalan, prednisone; VRd=bortezomib, lenalidomide, dexamethasone; VTd=bortezomib, thalidomide, dexamethasone.
References:
- Dimopoulos MA, et al. Ann Oncol 2021;32(3):309-322.
- Moreau P, et al. J Clin Oncol. 2019;37(suppl 15):8003.
- Voorhees PM, et al. Blood. 2020;136:936-945.
- McCarthy PL, et al. J Clin Oncol. 2017;35:3279-3289.
- Mikhael J, et al. J Clin Oncol 2019;37(14):1228-1263.
- Mateos MV, et al. Lancet. 2020;395:132-141.
- Facon T, et al. N Engl J Med. 2019;380:2104-2115.
- Devarakonda S, et al. Cancers 2021;13:863.