Chimeric antigen receptor T-cell (CAR-T) therapy is a promising treatment option for a broad range of cancers.1 The CAR-T therapies that have advanced the furthest in clinical trials are those targeting CD-19 expressing cells in acute and non-acute B-cell leukaemias and B-cell non-Hodgkin lymphomas.1 In 2020, the Australian government expanded its funding of access to the CAR-T therapy Kymriah (tisagenlecleucel) to include treatment for relapsed or refractory CD19-positive diffuse large B-cell lymphoma (DLBCL).2 Kymriah is also indicated for the treatment of B-cell precursor acute lymphoblastic leukaemia (B-ALL) in paediatric and adult patients (up to 25 years) that is refractory, in relapse post-transplant or in second or later relapse.3 These decisions were based in part on results of the JULIET trial – a global phase II trial of tisagenlecleucel in adult patients with DLBCL who had relapsed or were refractory to two or more prior lines of therapy.4 In late 2020, 40 month data from the JULIET trial was presented at the 62nd ASH Annual Meeting & Exposition, and in early 2021, preliminary data from a 60 month follow up was published in the New England Journal of Medicine. The limbic sat down with Dr Duncan Purtill, Consultant Haematologist and Director of the Blood and Marrow Transplant Programme at Fiona Stanley Hospital, Western Australia, to discuss the data and its implications for the management of DLBCL in Australia.
Initial outcomes – JULIET at 14 months
The pivotal JULIET trial was a global single-arm phase II study conducted across 27 sites in 10 countries.4 The study was designed to assess the safety and efficacy of tisagenlecleucel in adult patients with relapsed or refractory DLBCL, who were ineligible for or had disease progression after autologous haematopoietic stem-cell transplantation. The primary end point of the trial was best overall response rate.
In 2019, the first JULIET efficacy data from a median 14 months post-infusion was published. Of the 93 patients included in the efficacy analysis with three or more months of follow-up, or had discontinued participation in the study before three months, the best overall response rate was 52% (95% CI 41 to 62). Complete response was seen in 40% of patients and partial response in 12%. The rates of overall and complete response at month 3 were 38% and 32% respectively, and 33% and 29% respectively at month 6. In those who had a complete or partial response at month 3, the estimated progression-free survival (PFS) at 12-months was 83%.4
Consultant haematologist Dr Duncan Purtill commented on the short initial follow-up for JULIET, although data was collected from a reasonable number of patients. He noted that the trial population represents a small proportion of the overall DLBCL cohort, explaining “first line treatment is usually successful in 60–80% of patients, in whom relapse never occurs. Half of the remainder are cured by autologous stem cell transplant, leaving around 10–20% eligible for CAR-T therapy’. Bearing in mind that relapsed DLBCL is particularly difficult to treat, with a baseline expectation of progression free survival of 20% at 12 months, he commented that “the outcomes from the initial JULIET data look good, despite the short time-frame. Half of the study population responded to therapy and a significant proportion maintained that response, with complete response maintained up to 12 months later.”
In the context of this data, Dr Purtill noted the need to look at the “nitty-gritty’ details of CAR-T therapy – “it can take a reasonable amount of time to get onto a study before receiving CAR-T therapy. You must also be aware of time spent on chemotherapy and that the logistics of CAR-T manufacture can cause significant delays for patients with symptomatic disease. The longer the delay, the more likely we are to see disease progression.”
Follow-up – JULIET at 40 months
Presented at ASH 2020 and at Transplantation and Cellular Therapy (TCT) in February 2021, longer-term data from JULIET demonstrated the durability of the responses seen in the initial 14 month trial. Among all tisagenlecleucel responders, 60% maintained their response at both 24 and 36 months, with no new adverse events detected. 86% of those who were in complete response at 6 months were estimated to maintain that response at 36 months.
PFS was 33% and 31% at 24 months and 36 months respectively (Figure 1), with overall survival at 40% and 36%, respectively.5
Figure 1. Durable outcomes observed over a median of 40.3 months follow-up.5
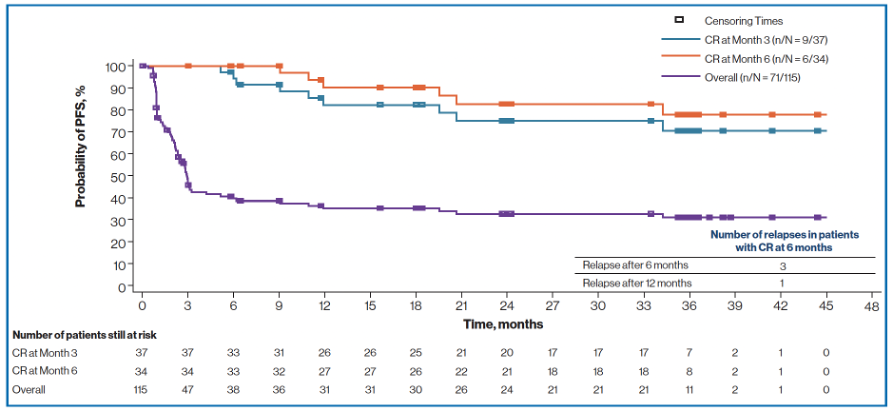
Note: efficacy assessments were performed at Day 28, Month 3, 6, 9, 12, 18, 24, 36, 48 and 60, or as clinically indicated. CR=complete response; DLBCL=diffuse large B-cell lymphoma; PFS=progression-free survival.
In patients who received lymphodepleting chemotherapy, the type of therapy appeared to predict the duration of progression-free survival, with a 24 month PFS of 38.2% in patients receiving fludarabine-based lymphodepleting chemotherapy, versus 14.1% in patients given non-fludarabine based therapy.5
Latest outcomes – 60 month data
In February 2021, Chong et al. reported 60 month follow-up data from the initial phase II single-centre study of tisagenlecleucel in lymphoma6, first published in 20177. At 5 years follow-up, 14 of the 24 DLBCL patients in this cohort had a complete or partial response (58%), with 11 demonstrating complete response (46%) at this time point. 31% of all patients remained progression-free, with a median PFS of 5.8 months (95% CI 1.6 to 64.2). Median duration of response was 61.4 months (95% CI 3.2 to could not estimate). At 5 years, 60% of responding patients continued to respond.6
The authors of this report acknowledge the small number of patients in this single-centre trial, concluding “most responses at 1 year were sustained at 5 years; however, some late relapses may occur”.6
Lessons learned from the long term data
The studies have highlighted the need to consider the practical challenges of CAR-T therapy. Firstly, when considering referral, Dr Purtill noted the need for careful patient selection – “a period of disease control is required prior to CAR-T to maximise the opportunity for infusion and response.” He also pointed out that toxicity should be expected, and that some patients may in fact not benefit from CAR-T. Referral for transplant is not required, as it may offer some disease control but not complete control.
Collection of CAR-T cells may be difficult in some patients. Dr Purtill advised that “most patients are receiving fludarabine containing regimens, and referrers should bear in mind that prior bendamustine treatment is associated with poor collection of CAR-T cells. Anti-CD19 therapy in general might preclude subsequent therapy if CD19 is no longer expressed on malignant cells at relapse.” Trials are currently underway for bi-specific antibodies in relapsed lymphoma, regardless of CD19 or CD20 expression. These therapies have long half-lives. “There is a recommendation not to attempt cell collection if antibody therapy for lymphoma has been given within the last 6 weeks” explained Dr Purtill. ‘Some centres choose to collect and store CAR-T cells prior to treatment with drugs with a long half-life in case subsequent CAR-T therapy is needed; this is decided centre by centre and requires capacity to store the cells.”
There remain several unmet needs regarding CAR-T therapy in DLBCL. Dr Purtill highlighted improving the identification of patients most likely to benefit from therapy. “Due to the high cost of treatment, it is better to identify as a priority those who are likely to respond.” He also raised the issue of steroid use for toxicity early after infusion, stating “this does not negate the efficacy of CAR-T cells. However, learning more about whether drugs could be used prophylactically or earlier in response to toxicities could help in keeping more patients out of hospital.”
Overall, Dr Purtill considered these long term data to demonstrate that tisagenlecleucel remains a good therapeutic option in DLBCL, even in complex patients who have failed multiple lines of therapy.
References:
- Neelapu SS, et al. Nat Rev Clin Oncol. 2018;15(1):47-62.
- MSAC Public summary document, Application No. 1519.1 – Tisagenlecleucel (CTL019) for treatment of relapsed or refractory diffuse large B-cell lymphoma. Available from: (DLBCL)http://www.msac.gov.au/internet/msac/publishing.nsf/Content/1519.1-public (accessed April 2021).
- Kymriah (tisagenlecleucel) Approved Product Information.
- Schuster SJ, et al. N Engl J Med 2019;380:45-56.
- Jaeger U, et al. Poster Presented at Transplantation and Cellular Therapy (TCT); February 8–12, 2021; P212. Previously presented at the 62nd ASH Annual Meeting & Exposition (ASH); December 5-8, 2020. P1194.
- Chong EA, Ruella M and Schuster SJ, N Engl J Med 2021;384:673–674.
- Schuster SJ, et al. N Engl J Med. 2017; 377(26): 2545–2554.