A second draft of MBS item numbers for colonoscopy has cut the number of new items to be introduced from 20 to 8 following strong pushback from gastroenterologists about excessive paperwork.
The new items, developed by the MBS Review Taskforce, had been scheduled to replace MBS items for colonoscopy 32090 and 32093 in March 2018 but implementation was delayed to allow for better alignment with new NHMRC Clinical Guidelines for Surveillance Colonoscopy due to be released in late 2018.
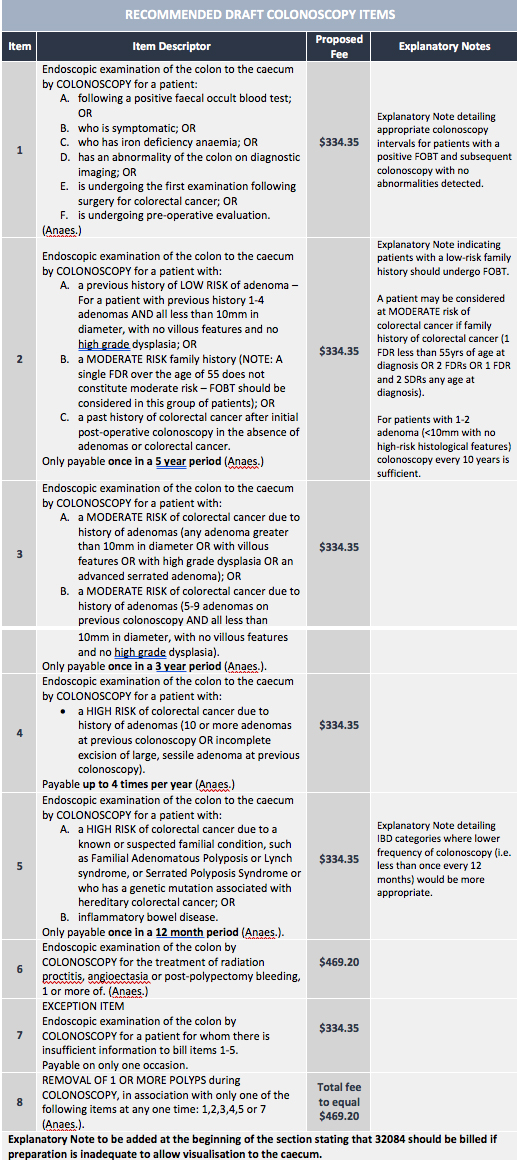
Following an August meeting with the Department of Health a new draft of eight items and has been released for consultation, according to GESA. The new items come with a basic fee of $334.35 for colonoscopic examination and a total fee of $469.20 for polyp removal.
The initial consultation also resulted in a statement on eight major changes to MBS items (see table).

In the feedback on the initial proposal for 20 items, the AMA raised concerns about using clinical guidelines in MBS item descriptions.
“Implementing guidelines directly into item descriptions risks placing arbitrary restrictions on services and may impede on expert contemporary clinical judgement and knowledge which should be applied to individual cases,” it said in a submission to the MBS Review taskforce.
The AMA also warned that the dramatic expansion of colonoscopy item numbers from 2 to 20 appeared to be for the purposes of gathering data, and would create an unnecessary paperwork burden for clinicians
“Whilst we note a key driver of the review is to reduce unnecessary medical services, increasing the number of service items may not have the intended result of reducing unnecessary colonoscopies, as there are foreseeable “shortcuts” that might be taken in order to minimise paperwork resulting in procedures being performed that may not fully meet the specified criteria,” it said.
According to the department of health, key considerations leading to the development of the new recommendations included:
- Acknowledgement that there is over-servicing of colonoscopy in some areas and underservicing in others but low rates of colonoscopy in some parts of Australia may not be addressed through modifications to the MBS.
- Items should be informed by clinical practice guidelines but not tied to them.
- Items should permit some flexibility for professional autonomy but clarify appropriate frequency of colonoscopy services for different clinical situations.
- In some instances, a clinician may not be reasonably able to obtain the information required to ensure clinical indications are met and a patient should not be disadvantaged by this.
The MBS Review Taskforce’s Clinical Committee is now evaluating feedback on the second draft of items and will then provide recommendations to the Federal Minister for Health.