Real-world evidence is beginning to play an increasing role in clinical decisions in a number of therapeutic areas.1 To understand the implications of these population health studies emerging in the diabetes space, the limbic spoke with Professor Jonathan Shaw, head of the Clinical and Population Health at the Baker Heart and Diabetes Institute.
Keeping it real: understanding the role of real world evidence
“It really comes down to understanding the benefits of clinical trials and real-world evidence. No one should be looking at just one or the other. Both types of information bring different things to the table,” explains Professor Shaw. “Real-world data is information collected outside of clinical studies. This may encompass large datasets of electronic health record information, disease registry information or other types of data relating to health status or the delivery of healthcare. It is important to realise that real-world evidence does not replace randomised controlled trials (RCTs). Real-world evidence has the potential to provide additional contextual information about patients and the delivery of patient care. While comparisons of interventions can be made using big data techniques such as propensity score matching, it has lost the vital component of a RCT – randomisation.”
Indeed, real-world data is a term that can apply to any information collected beyond what is normally collected in dedicated research studies.2 They provide a different type of contextual information about health and the delivery of care to RCTs.3 Undoubtedly, RCTs remain the gold standard for establishing efficacy – but due to the interventions being very specific and discrete (often one at a time) it can make establishment of effectiveness a challenge.3 This is where real-world evidence can lend a hand.2
Efficacy measures the effect of an intervention in an ideal setting. Effectiveness measures the effect of an intervention in ordinary clinical settings, where factors such as low adherence and costs may impact on the benefits of an intervention.3
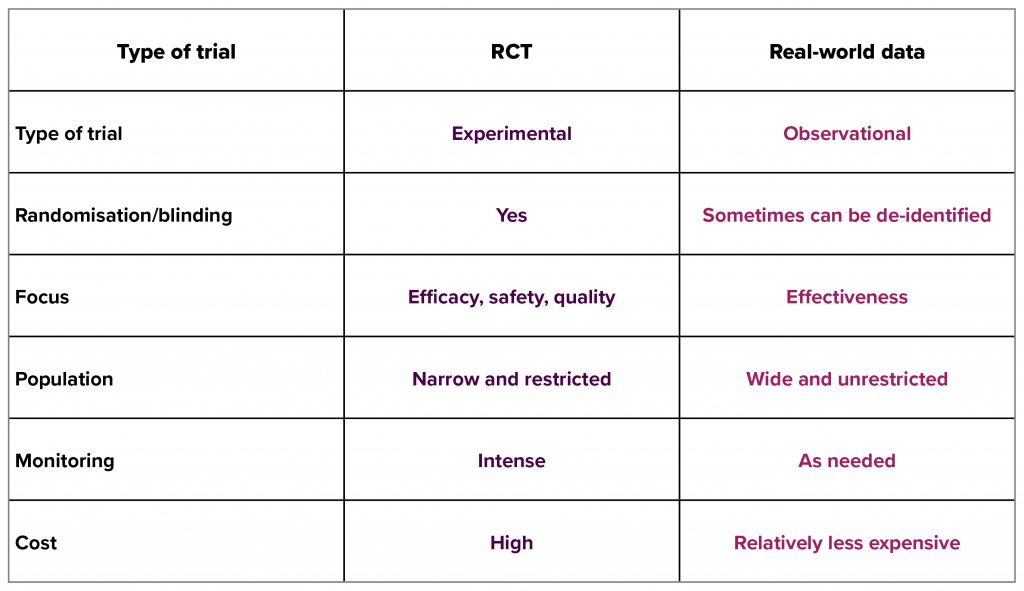
Adapted from Dreyer NA et al. 2018.4
Diabetes in the real world vs clinical trial
“Real-world studies are contributing to the evidence base we have in diabetes care. When analysed appropriately, they have the potential to provide additional information, such as how a medication works in populations not studied in a trial, how other factors such as treatment cost impact adherence to regimens or relative effectiveness compared with combinations of interventions not studied in clinical trials,” says Prof. Shaw.
“However, it is important to understand the limitations of real-world evidence and take them into consideration when we are interpreting their findings. Data may not be of as high quality as you find in clinical trials as the data may be incomplete, heterogeneous in its input or influenced by bias.2 Robust statistical methodologies must be followed to ensure you can confidently compare the outcomes of interventions in a heterogeneous population,” explains Prof. Shaw. “Propensity score matching is a common technique used in many real-world studies to minimise bias in the data.”5
Propensity score matching is a statistical matching technique used to estimate the effect of an intervention.5
Professor Shaw is an author on a recent publication of a large, international, retrospective, de-identified patient health record data that used propensity-matching to look at treatment outcomes in 235,064 patients who were initiated on a sodium-glucose cotransporter-2 inhibitor (SGLT-2i). The study confirmed clinical trial observations that initiation of an SGLT-2i was associated with a lower risk of CV events across a broad range of outcomes and patient characteristics.6 The authors concluded, “Collectively, our results suggest that the cardiovascular benefits of SGLT-2i may be applicable to a considerably broader patient population than previously considered. If further confirmed by the ongoing DECLARE-TIMI 58 trial, which is investigating the efficacy and safety of dapagliflozin in a broad population of patients with T2D, these findings could have substantial implications for clinical practice.”6
Indeed, studies like this, and similar ones like the DELIVER 2 study are able to examine the application of medications in clinical practice, helping to build a clearer picture of the overall benefit of an intervention, such as the prescription of insulin to a broader population base than is feasible to study in RCTs. DELIVER 2 study was a retrospective, observational study of electronic medical record data in 1,819 patients with type 2 diabetes switched from basal insulins to insulin glargine 300 Units/mL or another basal insulin.7 It demonstrated a lower incidence of hypoglycaemia and healthcare utilisation in patients switched to insulin glargine 300 Units/mL compared with those who switched to other basal insulins.7 “These results increase the body of evidence on the use of [insulin glargine 300 Units/mL] in people with type 2 diabetes from observational studies. While randomised clinical trials provide the highest level of evidence, comparative data from real-world observational studies can be relevant for payors and other organisations evaluating how findings from clinical trials can translate into routine clinical practice,” says Riccardo Perfetti, Head of Global Diabetes Medical Team at Sanofi.8
A similar observational study, LIGHTNING, examined 130,155 patients on long-acting insulins. Specifically, it compared patients on first generation basal insulin analogues (glargine 100 Units/mL and insulin detemir) and second genration basal insulin analogues such as insulin glargine 300 Units/mL and insulin degludec. “What these real-world studies are offering is the chance for us to compare many treatments, which for cost and logistics may never be possible to do in an RCT. Now we have a basis for understanding overall effectiveness in the clinical setting,” remarks Prof. Shaw.2,9
Keep one foot in the real world and the other in clinical trials
The way interventions are assessed has come a long way in recent years. As more and more real-world evidence is published from a variety of sources and centres, what is needed is a consensus on the appropriate design and curation of data to ensure integrity in the interpretations made.
A recent commentary by Dreyer et al. put forward a compelling argument for a framework to feed real-world evidence into regulatory frameworks:4
- Qualify RW data sources in the context of specific study objectives
- Apply intelligent designs using outcome measures based on real-world practices.
The authors note, “The outcomes measured, circumstances under which the data were recorded and populations covered should be placed in the context of other evidence and treatment options. The framework for assessing [real world] data quality must be grounded in the context of specific study objectives that are measurable in actual clinical practice. An agreed-upon framework will facilitate use of study-specific RW evidence as well as evidence derived from platforms that support repeated uses as follow-up accumulates and new questions arise.”4
Professor Shaw concludes, “It’s important to see the place for both real-world and RCT evidence. We will continue to require the strong internal validity an RCT provides, but also welcome the granularity and breadth real-world data offers.4 Now as clinicians, to ensure we have the best, most robust data to work with in the future, we should all be conscious about the quality and depth of information we record in patient records, as we might just come to rely on it for real-world studies in the future.”
This article was sponsored by Sanofi, which has no control over editorial content. The content is entirely independent and based on published studies and experts’ opinions, the views expressed are not necessarily those of Sanofi.
References:
- US Department of Health and Human Services. U.S. Food and Drug Administration. Science & Research. Real World Evidence. Available at: https://www.fda.gov/ScienceResearch/SpecialTopics/RealWorldEvidence/default.htm (accessed 7 June 2018).
- Mahajan R. Int J Appl Basic Med Res 2015;5(2):82.
- Young Kim S. Korean J Fam Med 2013;34:227.
- Dreyer NA. Ther Inn Reg Sci 2018;52(3):362-368.
- Ferdinand D et al. Int J Gen Med 2016;9:123-131.
- Kosiborod M et al. J Am Coll Cardioli 2018:2628-2639.
- Zhou FL et al. Diabetes Obes Metab 2018;20(5):1293-1297.
- Switching to Sanofi’s Toujeo® Showed Significant Reductions in Blood Sugar and Significantly Lower Incidence of Hypoglycemia in a Real-World Observational Study. April 2, 2017. Available at: http://www.news.sanofi.us/2017-04-03-Switching-to-Sanofis-Toujeo-Showed-Significant-Reductions-in-Blood-Sugar-and-Significantly-Lower-Incidence-of-Hypoglycemia-in-a-Real-World-Observational-Study (accessed 7 June 2018).
- Caffrey M. AMJC. In real-world study, Toujeo reduced risks of severe hypoglycaemia compared with older insulins. 14 February 2018. Available at: http://www.ajmc.com/newsroom/in-realworld-study-toujeo-reduced-risks-of-severe-hypoglycemia-compared-with-older-insulins (accessed 7 June 2018).