Hopes for a new class of oral anticoagulants targeting factor XI have been tempered by phase 2 study results showing they miss the ‘sweet spot’ of reducing thrombosis without increasing bleeding.
The much-anticipated results of small molecule oral inhibitors such as asundexian and milvexian have shown that while they inhibit factor XIa activity they did not meet their primary efficacy outcomes when used in patients with acute myocardial infarction and stroke.
Results of three key trials presented at the European Society of Cardiology 2022 meeting in Barcelona included the PACIFIC-AMI and PACIFIC-Stroke trials of asundexian and the AXIOMATIC Trials in patients with stroke or TIA.
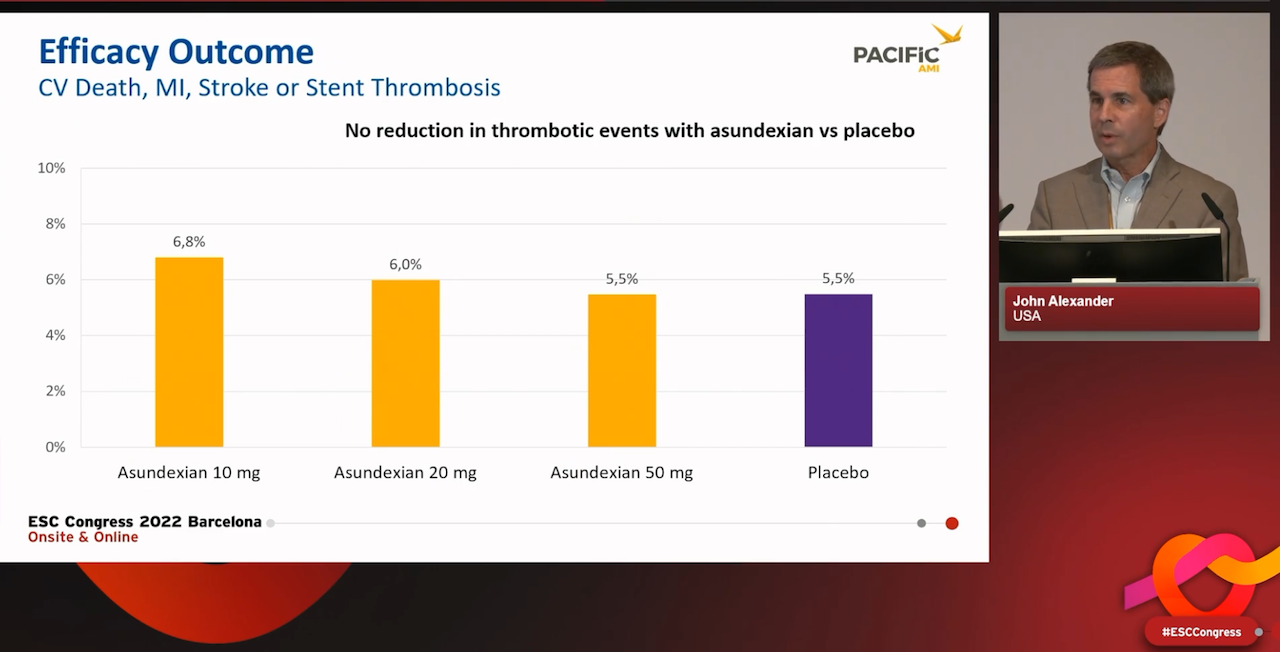
The PACIFIC-AMI study investigated three doses of asundexian (10, 20 and 50 mg daily) compared with placebo in more than 1600 patients treated with dual antiplatelet therapy (aspirin and a P2Y12 inhibitor) following an acute MI and PCI.
During one year of follow up asundexian produced a dose related reduction in factor XIa inhibition, with the 50 mg dose resulting in more than 90% inhibition.
There was no increase in bleeding with asundexian, which occurred in 7.6%, 8.1% and 10.5% of patients in the 10 mg, 20 mg, and 50 mg asundexian groups, respectively, and 9.0% patients in the placebo group.
However there was no reduction in ischaemic events, as assessed by the primary efficacy outcome of a composite of cardiovascular death, myocardial infarction, stroke, or stent thrombosis. This occurred in 6.8%, 6.0% and 5.5% of patients respectively, and 5.5% of patients in the placebo group.
The study lead investigator Professor John Alexander of Duke University School of Medicine, said the trial was primarily a dose finding study and was not powered to show conclusive results for efficacy. He said the results were promising and justified progression to larger studies.
“I wouldn’t call it unsuccessful in terms of efficacy results, they’re just inconclusive, and therefore it requires a subsequent phase 3 trial” he said.
A linked study, the PACIFIC-Stroke trial, failed to reach its primary endpoint for secondary prevention of non-cardioembolic ischaemic stroke with asundexian. However an exploratory analysis showed reduced recurrent ischaemic stroke and transient ischaemic attack (TIA) in some patients without increasing bleeding.
The phase 2 trial involved 1808 patients who received antiplatelet treatment and asundexian or placebo within 48 hours following an acute non-cardioembolic ischaemic stroke.
There was no apparent dose response effect on the composite of incident MRI-detected covert brain infarcts or recurrent symptomatic ischaemic stroke at six months.
During a median of 11 months of follow up the rate of recurrent symptomatic ischaemic strokes or TIA was 8.3% in the placebo group, 7.7% in the asundexian 10 mg group, 6.2% in the 20 mg group and 5.4% in the 50 mg group.
In a secondary exploratory analysis, recurrent ischaemic stroke or TIA was reduced among patients assigned to asundexian 50 mg compared with placebo (hazard ratio [HR] 0.64, 90% confidence interval [CI] 0.41–0.98), with the largest reduction among those with extracranial or intracranial atherosclerotic plaque (HR 0.39, 90% CI 0.18–0.85).
There was no increase in bleeding with asundexian compared to placebo at 12 months, and reassuringly this included haemorrhagic transformation, the study investigators said
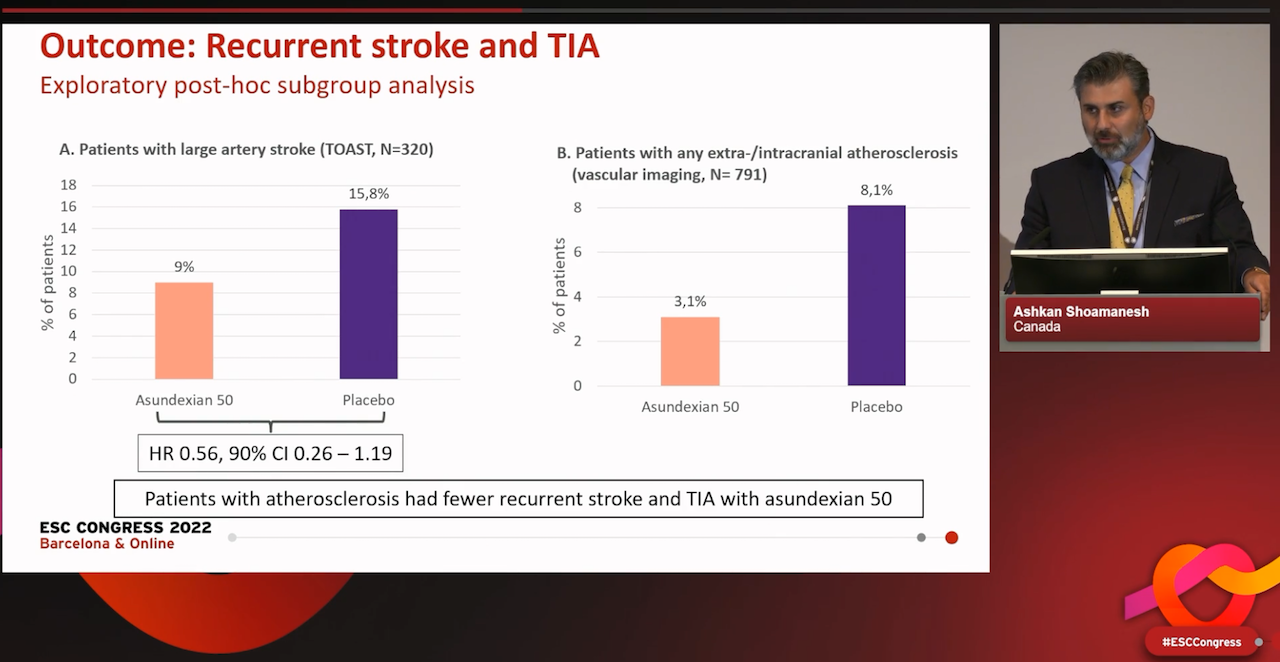
Lead investigator Dr Ashkan Shoamanesh of the Population Health Research Institute, Hamilton, Canada, said the negative results for the overall outcome appeared to be driven by a lack of effect on covert brain infraction due to small vessel disease, while the reduction in stroke and TIA was seen particularly among patients with atherosclerosis.
“The results of PACIFIC-Stroke indicate that the potential of asundexian to prevent stroke in selected patients should be investigated further,” he said.
“The promising results from this phase 2 trial require validation in an adequately-powered phase 3 randomised trial before being applied to clinical care for secondary stroke prevention.”
A similar call for phase 3 studies was made by the investigators of the phase 2 AXIOMATIC-SSP trial, which assessed the effects of the Factor XIa inhibitor milvexian on stroke occurrence and bleeding in patients with a high risk of recurrent stroke and associated disability.
The study enrolled 2,366 patients in 27 countries, who received one of five different doses of milvexian or placebo daily for 90 days in addition to background treatment with open-label aspirin and clopidogrel for 21 days followed by aspirin only.
While the rate of the primary efficacy endpoint was numerically lower with some doses of milvexian, there was no apparent dose-response. Milvexian reduced the risk of clinical ischaemic stroke (excluding covert brain infarction) in the intention-to-treat population at all doses except 200 mg twice daily, with doses from 25 to 100 mg twice daily showing an approximately 30% relative risk reduction versus placebo.
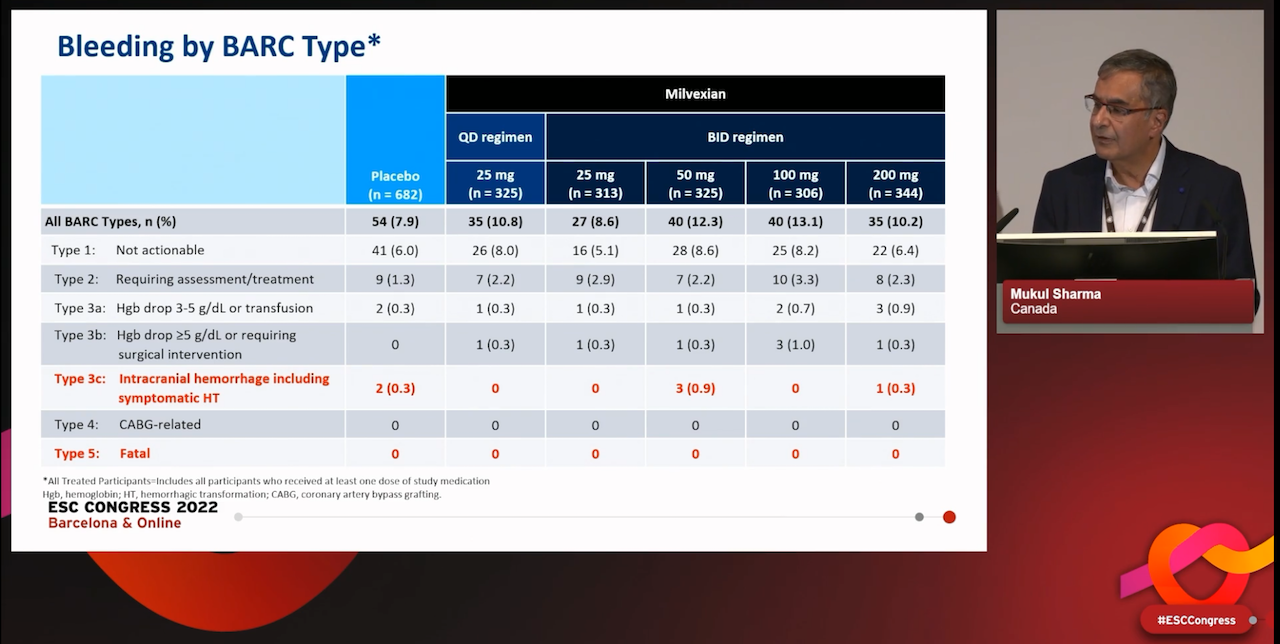
The incidence of major bleeding was low overall and the rate of major bleeding for the milvexian 25 mg once daily and twice daily doses was similar to placebo, while a moderate increase was observed in the milvexian dose arms of 50 mg twice daily and above, with no apparent dose-response. There was no increase in severe bleeding (BARC type 3c or symptomatic intracranial haemorrhage) versus placebo, and there was no fatal bleeding in any arm of the study.
Principal investigator Dr Mukul Sharma of McMaster University, Hamilton, Canada, said it was again notable that factor XI inhibition did not appear to affect covert infarcts.
Milvexian will now be further studied in a phase 3 trial in a similar stroke population, he told the meeting.