Many patients fail to reach their lipid treatment goals despite best standard-of-care treatment.1,2 New therapies are needed to address residual cardiovascular risk, particularly in high-risk and very high-risk dyslipidaemia. Ribonucleic acid (RNA)-based therapies developed for genetically validated targets are potentially cost-effective, simple to manufacture and are showing promising results in clinical trials.3 The limbic spoke with Professor Gerald F Watts, Winthrop Professor of Cardiometabolic Medicine, School of Medicine, University of Western Australia, and Head of the Cardiometabolic Service, Departments of Cardiology and Internal Medicine, Royal Perth Hospital, to learn more about how this emerging novel class of therapeutics could impact clinical practice in cardiovascular medicine.
Unmet needs for patients with dyslipidaemias
Atherosclerotic cardiovascular disease (ASCVD) is a global leader in cause of death and disability.4 In Australia, ASCVD causes 1 in 6 adult deaths and over 250,000 hospitalisations each year.5 Abnormal lipids play a central role in ASCVD and are a major risk factor in cardiovascular events.6
Current pharmacological management of dyslipidaemias centres on statin therapy.7,8 Most high-risk patients fail to reach their guideline-recommended low-density lipoprotein cholesterol (LDL-C) treatment goal on moderate or high-intensity statins however, and are at high risk of recurrent and fatal cardiovascular events.1,2 Issues with adherence and tolerability and a high pre-treatment LDL-C – in severe familial hypercholesterolaemia for example – can impair the ability to reach guideline-recommended targets with standard therapies. Much of the residual risk that remains after statin therapy can be attributed to disordered lipid and lipoprotein metabolism (so called dyslipoproteinaemia), and lowering LDL-C has been shown to directly and proportionally reduce major ASCVD events.3,9 Newer antibody-based therapies, such as the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors evolocumab and alirocumab, are effective in further lowering LDL-C levels, particularly when used in combination with high-intensity statins.7 However, antibody-based therapies are not feasible for targeting proteins without enzyme activity, such as lipoprotein(a) (Lp(a)) and apoC3.3
Other important and independent contributors to cardiovascular risk include elevated triglycerides (TG), elevated Lp(a) and triglyceride-rich lipoprotein (TGRL) remnants.3,4 “Elevated LDL-C, Lp(a) and TGRL have interdependent and cumulative effects but they are independent risk factors with separate genetic control, so it is important to identify and treat the presence of each one”, stressed Prof Watts. In those with lipid abnormalities beyond LDL-C, such as those with familial combined hyperlipidaemia, familial hypertriglyceridemia and atherogenic dyslipidaemia related to type 2 diabetes, substantial residual risk persists, despite treatment with currently available lipid-modifying medications.4
Prof Watts also points out that “other factors such as inflammation, uncontrolled diabetes, uncontrolled hypertension and thrombosis also contribute to residual risk of ASCVD – and are important to consider.”
Why explore RNA therapeutics?
Less than 15% of human proteins have active binding sites that can act as drug targets – the majority are ‘undruggable’ by conventional therapies.10 Recombinant proteins and peptides have expanded druggable targets, but have their own challenges in terms of size, stability and complex manufacturing.10
The variety and abundance of RNAs and their involvement in diverse cellular functions, including gene expression, opens up their potential for use in medicine.11,12
The potential to design RNA therapies against virtually any genetic target with greater specificity than small molecules opens up the possibility of addressing a broad range of diseases with minimal drug-drug interactions.13 RNA-based medications are potentially cost-effective and quick to manufacture compared to small molecules or recombinant proteins, and can also be rapidly altered for personalised treatment.10 Notable recent examples are Nusinersen, the first treatment for the rare and often-fatal spinal muscular atrophy.14
Volanesorsen is the only RNA therapeutic approved for use in a lipid disorder, although it is not approved in Australia. Volanesorsen can improve outcomes in patients with Familial Chylomicronemia Syndrome (FCS) and is under investigation for familial partial lipodystrophy.3,10 Many more RNA therapeutics are also in development for dyslipidaemias – and are showing promising results in clinical trials.3,10
RNA and RNA therapeutics – background
RNA therapeutics target messenger RNA (mRNA), but are categorised according to their differing mechanisms of action – antisense oligonucleotides (ASO), RNA interference (RNAi) therapeutics and messenger RNA (mRNA) therapeutics.10
ASO are short strings of synthetic single-stranded nucleic acids, mostly DNA based, designed to bind to the target mRNA. The RNA-DNA duplex is degraded via endogenous RNase H enzymes, with the ultimate effect of blocking synthesis of the functional protein.3,10
siRNA and miRNA are small double-stranded RNA that elicit RNAi – a conserved biological process that results in destruction of target mRNA in response to double-stranded RNA, and thus, post-transcription gene silencing.3,10 The mechanism of action of siRNA and miRNA is similar but with a notable difference – siRNA destroys one specific target gene, whereas miRNA can regulate the expression of multiple genes.10
mRNA therapeutics have arguably the simplest mechanism of action. Exogenous mRNA is introduced into the host cell where it is translated into the encoded protein or antigen.10 Synthetic mRNA is often used in replacement therapy to compensate for a defective gene/ protein or to supply therapeutic proteins.10 Vaccines can also use mRNA encoding specific antigen(s) to elicit immunity against the antigens.10 The Pfizer-BioNTech and Moderna COVID-19 are mRNA-based.
RNA therapies for dyslipidaemias
Several RNA therapeutics are currently in development for dyslipidaemias. The drugs differ in their mechanism of action and primary lipid or lipoprotein targets.
Most of the pipeline RNA therapeutics for dyslipidaemias employ a drug target that is genetically validated in humans. Prof Watts explains why this is important: “By ‘genetically validated’ we mean that we have genetic evidence that these genes are causative of lipid imbalances and cardiovascular disease in humans. People with naturally occurring loss-of-function mutations in genes such as APOC3 and ANGPTL3 show reduced risk of ASCVD with no major side effects. These natural variants demonstrate that there are benefits in lowering or knocking out the proteins encoded by these genes, and that this can be achieved safely. Essentially, this is what we’re essentially trying to recapitulate or recreate with the RNA therapeutics.”
Here, we review some of the current and upcoming RNA-based therapies for dyslipidaemias, including their efficacy, safety and current development status.
Inclisiran lowers LDL-C
Inclisiran inhibits the transcription of PCSK9 – a proprotein convertase that promotes degradation of LDL receptors. The drug is a long-acting siRNA with an N-acetylgalactosamine (GalNAc) conjugation, and works intracellularly to cause degradation of PCSK9 mRNA and inhibit its synthesis.3 This results in increased expression of LDL receptors on liver cells, promoting uptake of circulating LDL-C, thereby decreasing plasma LDL-C levels.15 Figures 1 and 2 show the mechanism of action of inclisiran in hepatocytes.
Figure 1. GalNAc conjugation enables rapid and specific uptake of inclisiran into hepatocytes via the asialoglycoprotein receptor (ASGPR) system. 1. GalNAc-conjugated inclisiran binds with high specificity and affinity to ASGPR on the hepatocyte cell surface. 2. Endocytosis and delivery of the complex to the endosome is mediated by clathrin-coated vesicles. 3. Acidification of the endosome promotes dissociation of the complex and degradation of GalNac. 4,5. ASGPR is then recycled rapidly to the cell surface with inclisiran released into the cytoplasm of the hepatocyte.
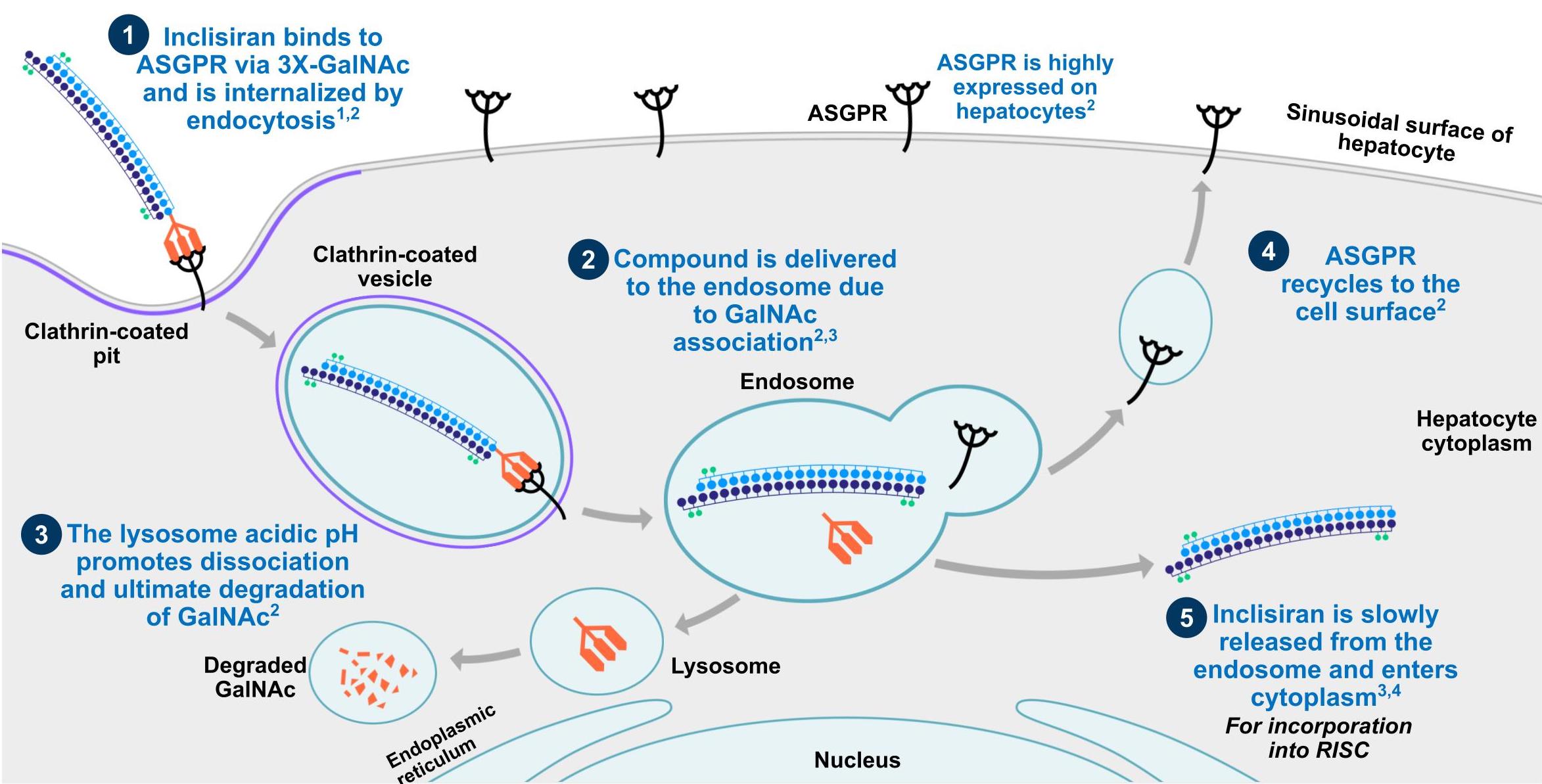 1. Wang N et al. Circ Res. 2017;120:1063-1065. 2. Springer AD et al. Nucleic Acid Ther. 2018;28:109-118. 3. Khvorova et al. N Engl J Med. 2017;376:4-7. 4. Tsouka AN et al. Curr Pharm Des. 2018;24:3622-3633.
1. Wang N et al. Circ Res. 2017;120:1063-1065. 2. Springer AD et al. Nucleic Acid Ther. 2018;28:109-118. 3. Khvorova et al. N Engl J Med. 2017;376:4-7. 4. Tsouka AN et al. Curr Pharm Des. 2018;24:3622-3633.
Figure 2. Inclisiran directs the RISC to cleave the target mRNA of PCSK9, preventing production of the PCSK9 protein within the cytoplasm of the hepatocyte. 1,2. Inclisiran in the hepatocyte cytoplasm binds to the RNA Induced Silencing Complex (RISC), a multi-enzyme complex that incorporates the strands of siRNA. 3. The passenger (sense) strand of inclisiran is cleaved away and degraded, leaving the guide (anti-sense) strand bound to the RISC. 4. The guide strand directs the RISC to the mRNA of PCSK9. 5-8. Complementary base pairing between the RNA strands leads to RISC-mediated catalytic cleavage of the PCSK9 mRNA, resulting in no synthesis of PCSK9 protein.
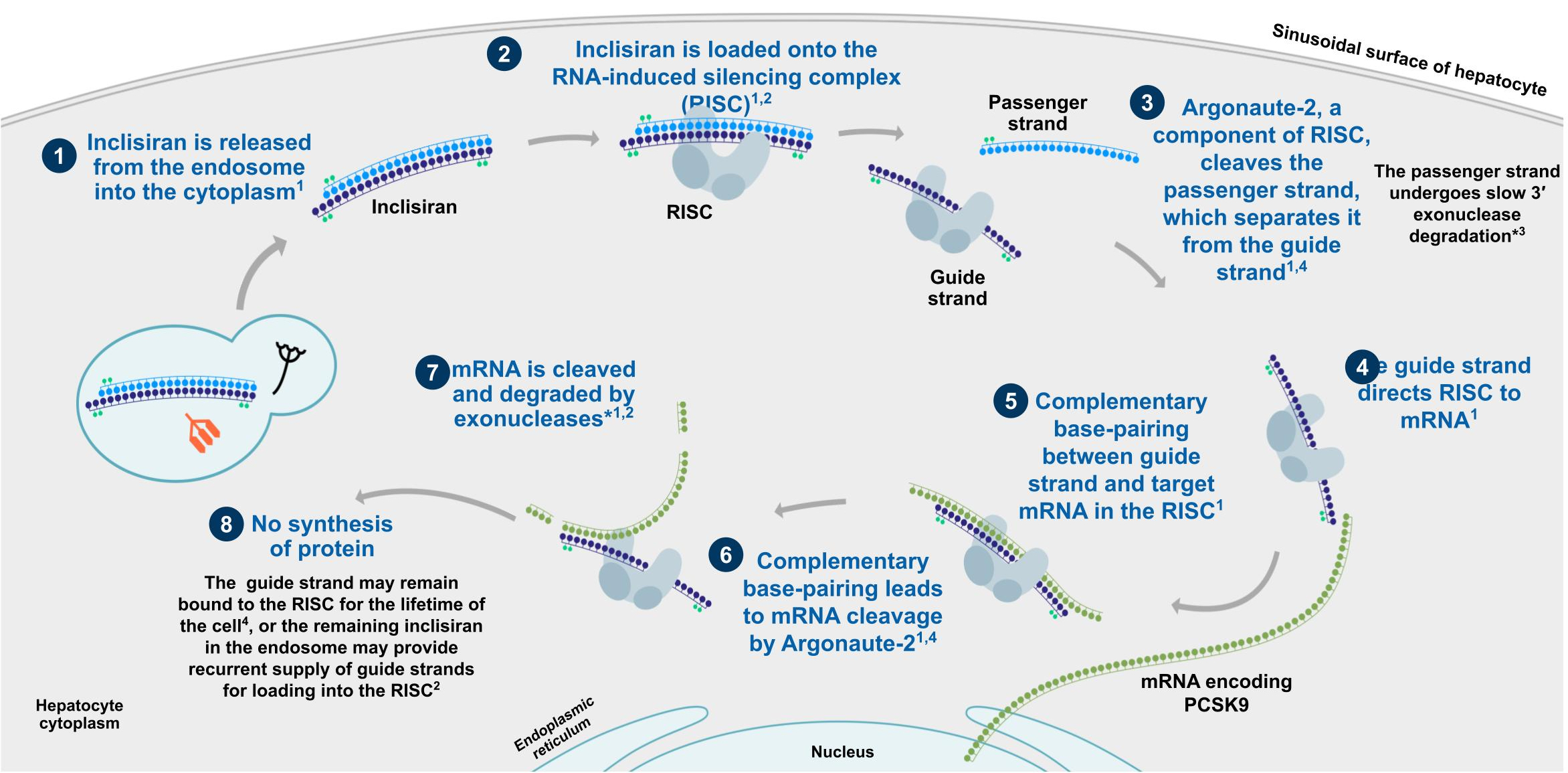 1. Tsouka AN et al. Curr Pharm Des. 2018;24:3622-3633. 2. Khvorova A et al. N Engl J Med. 2017;376:4-7. 3. Novartis Pharmaceutical Corp; 2018. 4. Rand TA et al. Cell. 2005; 123:621-629.
1. Tsouka AN et al. Curr Pharm Des. 2018;24:3622-3633. 2. Khvorova A et al. N Engl J Med. 2017;376:4-7. 3. Novartis Pharmaceutical Corp; 2018. 4. Rand TA et al. Cell. 2005; 123:621-629.
In phase 2 trials in patients with ASCVD, inclisiran reduced LDL-C by up to 53% at 6 months, following a 300mg two-dose regimen (300mg administered at day 1 and day 90).3 In addition, non-high-density lipoprotein C (non-HDL-C) was reduced by 46% and apolipoprotein B (apoB) by 41% (P<0.001 for all groups versus placebo).3 The presence of diabetes or renal dysfunction did not appear to impact efficacy and no dose adjustment was needed in patients with renal impairment.3 Phase 3 trials in patients with ASCVD (and risk equivalent) and HeFH have been completed.15,16 In HeFH, inclisiran demonstrated a significant reduction in LDL cholesterol, with a 47.9% difference between treatment and placebo groups at day 510 (P<0.001 vs. placebo).15 In trials of patients with ASCVD (ORION-10) and ASCVD/ASCVD risk equivalent (ORION-11), inclisiran reduced LDL cholesterol by 52.3% and 49.9%, respectively at day 510 (P<0.001 vs. placebo).16 A longer term cardiovascular outcomes trial is currently underway.3
A pilot study of the 300mg two-dose regimen of inclisiran in 4 patients with homozygous familial hypercholesterolaemia (HoFH) showed a 30% reduction in LDL-C, which lasted up to 6 months in 3 of the patients.3 A larger study in 45 HoFH patients is underway to confirm these results. The long-lasting effect of inclisiran on LDL-C has led to a proposed 6-monthly dosing schedule.3
Therapies targeting Lp(a)
Lp(a) is composed of an LDL-like particle bound to the apoprotein apo(a). The addition of apo(a) renders Lp(a) more atherogenic, on an equimolar basis, than LDL.4 This is because Lp(a) has additional pro-inflammatory, pro-oxidative, pro-thrombotic and anti-fibrinolytic properties. Elevated Lp(a) is associated with increased risk of coronary artery disease in both epidemiological and genetic studies.4 PCSK9 mAbs reduce Lp(a) by 25–30%, but there are currently no pharmacological treatments that effectively target Lp(a) reduction.4
While the mechanism of Lp(a) clearance from plasma is not well understood, up to 90% of the variability in plasma Lp(a) concentration is genetically determined3, which essentially drives the hepatic synthesis and secretion of Lp(a) particles into the circulation. A nucleic acid-based therapeutic that targets synthesis of apo(a) and thereby synthesis and secretion of Lp(a), appears therefore to be a feasible treatment approach.
Pelacarsen (APO(a)-LRx), a GalNAc-conjugated ASO, blocks synthesis of apo(a) via degradation of its encoding mRNA.3 In a phase 2b dose-ranging trial in 286 patients with pre-existing cardiovascular disease and elevated baseline Lp(a) >60mg/dL, pelacarsen dramatically reduced plasma Lp(a) – up to 80% mean reduction with 20mg weekly dosing. LDL-C and apoB were lowered by 21% and 15%, respectively.3 Pelacarsen was well tolerated, with no safety concerns related to platelet count, liver or renal function, and no signs of influenza-like symptoms. Injection site erythema was the most commonly reported adverse event (25%).3
A pivotal phase 3 trial of pelacarsen versus placebo [Lp(a)HORIZON; NCT04023552] is currently recruiting approximately 7,680 patients with ASCVD who have controlled LDL-C and elevated Lp(a) ≥70mg/dL.17 The study will further investigate causality between elevated Lp(a) and major cardiovascular outcomes, as well as further investigate efficacy and safety of pelacarsen in lowering Lp(a) and improving outcomes. The study is estimated to be completed in mid 2024.17
AMG890 (Olpasiran) is another RNA-targeted Lp(a) lowering therapy currently in early clinical development. AMG890 is a GalNAc-conjugated siRNA targeting mRNA encoding the LPA gene, and is currently in a phase 2 trial anticipated to be completed early 2023. Phase 1 data has shown significant reductions in Lp(a), with a single dose reducing mean Lp(a) by 71-96% at day 43, and by 80-94% at day 113, depending on dose. AMG890 was well-tolerated, with no unexpected safety concerns.18
Therapies targeting TGs: volanesorsen and vupanorsen
Volanesorsen (APOC3I-Rx) is an ASO that specifically binds mRNA of the apoC-III encoding gene APOC3, promoting its degradation and lowering production of apoC-III, a key regulator of the hepatic production and peripheral clearance of TGs.3 Carriers of loss-of-function mutations of APOC3 show low levels of serum TG and a reduced risk of myocardial infarction and ischemic vascular disease.3 On this basis, apoC-III appears to be a rational target for future therapeutics for FCS.3
Volanesorsen is the only RNA therapeutic currently approved (in the EU) for use in a lipid disorder, although it is not approved in Australia. Volanesorsen may improve outcomes in patients with FCS.3,10,19,20 After 3 months of volanesorsen therapy, plasma apoC-III levels in patients with FCS were reduced by more than 80% from baseline.3,19 Mean TG levels were reduced by 73–77% and acute pancreatitis events were significantly lower (1 event) than placebo (9 events in 6 patients).3 Conversely, a rise was observed in LDL-C (136%) and HDL-C (46%). Most patients given this type of volanesorsen, that was not ligand-conjugated and therefore not liver specific, experienced a gradual and mild decline in platelet counts and some experienced rapid and unpredictable severe thrombocytopenia requiring hospitalisation or treatment.3
Subsequent to the above trials, a GalNAc conjugation of volanesorsen has improved the safety profile of the resulting formulation (APOCIII-LRX), with no platelet count reductions seen in healthy subjects with elevated TG. Phase 3 data of GalNAc-conjugated volanesorsen in patients with FCS or multifactorial severe hypertriglyceridaemia and a fasting plasma TG ≥ 500 mg/dL demonstrated sustained reductions in TG after 6 months of treatment, compared to placebo.21,22An ongoing phase 2 trial is enrolling patients with hypertriglyceridemia and established ASCVD.3
Until the 2019 approval of volanesorsen in Europe, the only effective therapeutic approach for FCS was extreme restriction of dietary fat – a difficult task when life-long compliance in required.19 Based on positive outcomes in FCS patients, volanesorsen is now being evaluated in patients with familial partial lipodystrophy.3
Another GalNAc-conjugated ASO targeting TG, vupanorsen (ANGPTL3-LRx), is also in the pipeline. Vupanorsen targets ANGPTL3 mRNA and reduces production of ANGPTL3, a hepatic protein involved in inhibition of lipoprotein lipase and endothelial lipase.3 People with an ANGPTL3 loss-of-function mutation show lower TG, LDL-C and HDL-C, reduced cholesterol content in TGRL and their remnants, as well as lower risk of cardiovascular disease and myocardial infarction.3
In a phase 2 dose-ranging trial in patients with elevated TG levels, type 2 diabetes and non-alcoholic fatty liver disease, an 80mg every 4 weeks dose of vupanorsen reduced ANGPTL3 by 62%, leading to a 53% reduction in TG. After 6 months on treatment, half of patients had reached TG levels <150 mg/dL.4 Vupanorsen also reduced apoC-III (58%), remnant cholesterol (38%) and non-HDL-C (18%). Injection site redness and itching were the most frequent adverse events and no patients experienced a platelet count <100,000/mm.3,4
In development: ARO-ANG3
ARO-ANG3 is an investigational RNAi drug that induces silencing of the ANGPTL3 gene in the liver while avoiding off-target effects. Initial phase 1/2a data in healthy volunteers who received a single ascending dose of ARO-ANG3 showed dose-dependent reductions in fasting TG, LDL-C and HDL-C and a favourable safety and tolerability profile.23 Duration of effect of at least 12 weeks after a second dose was subsequently demonstrated in normal subjects, with good tolerability over 16 weeks.24 In 2020, preliminary results in 17 people with HeFH receiving open label subcutaneous ARO-ANG3 at repeat doses of 100, 200 or 300mg (days 1 and 29) were reported, and showed consistently reduced LDL-C (23-37%) and TG (25-43%) at all doses.25 Non-FH patients with high LDL-C ( >70mg/dl despite statin therapy) also included in this trial showed mean reductions in LDL-C and TG of 28% and 29% respectively. Most AEs were mild (30% of subjects with respiratory tract infections), with no drug-related serious adverse events or discontinuations reported.25 ARO-ANG3 is currently the subject of a double blind, placebo-controlled phase 2b trial in patients with mixed dyslipidaemia.26
Optimising RNA therapies through chemical modification
The therapeutic use of RNA-based therapies was initially challenging.
Unmodified ASO are susceptible to nuclease degradation, have poor protein binding and therefore inefficient tissue uptake.20 Many chemical modifications have been developed to improve the pharmacokinetic and pharmacodynamics properties of ASO.20 Second- and third-generation ASOs include modifications that improve their stability, resistance to nucleases, target specificity, binding affinity to target mRNA, bioavailability, and lower their toxicity.3,20
In contrast, the optimisation of siRNA has focused on improving tissue targeting and cellular uptake.3 Unmodified siRNA do not bind to serum proteins and are rapidly excreted, so additional measures are needed to ensure effective tissue delivery.13 Examples of such modifications include formulating the siRNA in lipid nanoparticles or chemical conjugation to a moiety that interacts with a target-cell surface receptor to facilitate cell uptake.13
Conjugation with GalNAc moieties allows therapeutics to be targeted to hepatocytes.13 Triantennary GalNAc is a ligand that binds with high affinity to asialoglycoprotein (ASGPR) receptors found specifically in liver cells.3 GalNAc conjugation allows targeted delivery to and uptake of an ASO or siRNA by liver cells via the ASGPR receptors, and is currently most widely used in RNA therapeutics aimed at treatment of hyperlipoproteinemias.3
“GalNAc conjugation has been a major leap forward for RNA therapeutics,” noted Prof. Watts. “By improving specificity for hepatic drug targets, it has made a vast amount of difference in toxicity. GalNAc conjugation also permits use of much lower doses.”
The first formulation of an siRNA targeting PCSK9 synthesis, ALN-PCS, had inadequate duration of effect – a major setback for a therapeutic that was showing otherwise promising reductions in PCSK9 and LDL-C.3 The development of inclisiran, ALN-PCS functionalised with GalNAc, improved duration of action to beyond 3 months.3 APOCIII-LRx, a GalNac-conjugated formulation of volanesoren, did not show platelet count reductions seen with volanesoren.3 The addition of GalNAc to APO(a)Rx, forming APO(a)-LRx or pelacarsen, improved effectiveness by more than 30 times, allowing for greatly reduced doses.3
RNA therapeutics for dyslipidaemias – where to next?
RNA therapeutics have very compelling potential in medicine, but many hurdles must be overcome before their widespread use in clinical practice. Prof Watts acknowledges their great potential in the treatment of dyslipidaemias and unaddressed questions.
“Nucleic acid-based therapies overcome some of the shortcomings of previous pharmacological agents. Several appear to be excellent in short-term trials – specific, potent and durable. But RNA therapeutics are the ‘new kids on the block’. Experience with them is limited and we need to confirm long-term safety and efficacy, including their impact on cardiovascular disease outcomes; this is evidently essential before they can be used in clinical practice.”
Prof Watts also emphasises that efficacy is important but safety takes precedence. “Safety has not been investigated in children, pregnant women, different ethnic groups and patients with hepatic impairment or disease. Many other safety questions also need to be answered. For example, what type of off-target adverse effects might occur, particularly in patients with liver disease? Could the durability of siRNA agents have a downside, whereby any adverse effects are also long lasting? We need to know about toxic effects and how to reverse them should they occur. But so far the trial results have been very reassuring about the safety and tolerability of these new therapeutic agents.”
Prof Watts points out that we are also yet to determine the ideal patients for the various RNA therapeutics being developed for dyslipidaemias. “For high- and very high-risk patients, such as those with severe FCS or HoFH, the indications and benefit of these therapies may be clear, but what about patients with mild forms of dyslipidaemia? Is there a role for these treatments in patients with mildly or moderately elevated plasma lipid levels who are currently receiving best standard of care or are intolerant to statins, and who may be at lower absolute risk of ASCVD? Will they be cost effective and affordable?”
“We certainly need more information on safety and tolerability of these agents, but once that’s confirmed, RNA-based therapies could have a major role in addressing residual risk of ASCVD in many high risk patients. Successful development of RNA therapeutics will address major unmet needs in the management of dyslipidaemias, particularly the shortcomings of current agents to manage elevated TGRL and Lp(a). These new therapies could allow patients to achieve targets for LDL-C, Lp(a), and TGRL in an effective, durable and potentially safe way; this is most relevant to patients with FH, very high Lp(a), diabetic patients with residual HTG, and severe FCS. RNA therapeutics could also overcome the problems arising from drug intolerance, poor drug adherence and persistence, and the inconvenience of having to remember to take many tablets or dozens of injections a year.”
To conclude, Prof Watts emphasises the need to ensure that the new drugs are cost effective so that all patients who need them, especially disadvantaged patients, can access them, going on to point out that “There is also the obstacle regarding awareness and education faced by very new therapies. Educating and empowering patients so they can make informed decisions, and educating clinicians so they can make effective shared decisions with their patients – these are critical issues for the success and impact of these exciting new drugs.”
References:
-
- Fox KM et al. Clin Res Cardiol 2018;107:380–88.
- Jernberg T et al. Eur Heart J 2015;36(19):1163-70.
- Macchi C et al. Pharmacol Res 2019;150:104413.
- Ruscica M et al. Curr Atheroscler Rep 2021;10;23(5):17.
- Australian Institute of Health and Welfare. Web report: Cardiovascular disease. CVD 88. 2020. Available at: https://www.aihw.gov.au/reports/heart-stroke-vascular-disease/cardiovascular-health-compendium/contents/how-many-australians-have-cardiovascular-disease. Accessed June 2021.
- Yusuf S et al. Lancet 2004;364:937-52.
- Mach F et al. Eur Heart J 2020;41:111–188.
- National Vascular Disease Prevention Alliance. Guidelines for the management of absolute cardiovascular disease risk. 2012.
- Ference BA et al. Eur Heart J 2017;38:2459–72.
- Damase TR et al. Front Bioeng Biotechnol 2021;9:628137.
- Clancy S. Nature Education 2008;1(1):102.
- Morris K. Semin Cell Dev Biol 2011;22(4):351–358.
- Crooke ST et al. Cell Metab 2018;27(4):714-739.
- NPS MedicineWise. Aust Prescr 2019;42:75–6.
- Raal FJ et al. N Engl J Med 2020; 382: 1520-1530.
- Ray KK et al. N Engl J Med 2020; 382: 1507-1519
- ClinicalTrials.gov. Assessing the Impact of Lipoprotein (a) Lowering With TQJ230 on Major Cardiovascular Events in Patients With CVD (Lp(a)HORIZON). Available at: https://clinicaltrials.gov/ct2/show/NCT04023552. Accessed July 2021.
- Koren MJ. Circulation 2020;142:A13951.
- Gaudet D et al. N Engl J Med 2014;371(23):2200-6.
- Scoles DR et al. Neurol Genet 2019;5(2):e323.
- Gouni-Berthold I et al. Lancet Diab Endocrin. 2021:9;264-275.
- Watts GF, Ward NC. Lancet Diab Endocrin. 2021:9;248-249.
- Watts GF et al. Abstract 20952. American Heart Association’s Scientific Sessions 2019 – late breaking abstracts. Circulation 2019;140:e964.
- Watts GF, et al. European Heart Journal 2020; 41(Supp 2).ehaa946.3331.
- Watts GF et al. Abstract 15751. American Heart Association’s 2020 Scientific Sessions. Circulation. 2020;142:A15751.
- ClinicalTrials.gov. Study of ARO-ANG3 in Adults With Mixed Dyslipidemia. Available at: https://clinicaltrials.gov/ct2/show/NCT04832971. Accessed 28 June 2021.