With an expanded eligibility criteria announced earlier this year, evolocumab (Repatha) can now be prescribed for patients with or without familial hypercholesterolaemia (FH). To be eligible for treatment non-FH patients must have symptomatic atherosclerotic cardiovascular disease (ASCVD) and at least one additional risk factor and must have been optimally treated for more than 12 weeks with the maximum statin dose they can tolerate plus ezetimibe.1-3
The practical application of the expanded PBS criteria in the clinic
In a series of webinars held during the COVID-19 lockdown, Amgen aimed to upskill specialists in optimal lipid management in high-risk ASCVD patients. The first webinar in the series was chaired by Professor Steve Nicholls, Director of MonashHeart and Director of The Victorian Heart Institute at Monash University, Melbourne, and featured Professor Ann Marie Navar, Professor of Medicine, Division of Cardiology, University of Texas Southwestern Medical Center, USA, as well as local experts Professor Karam Kostner from the University of Queensland and Director of Cardiology at the Mater Hospital, Brisbane, and Professor Clara Chow, Professor of Medicine at the University of Sydney and Academic Director of the Westmead Applied Research Centre, Sydney. This was followed by five case study webinars each of which covered a case relevant to the new PBS listing for evolocumab (Repatha). Here’s what happened.
Unmet needs of high-risk cardiovascular patients with elevated LDL-C
For patients with ASCVD as many as 8 in 10 are not achieving low-density lipoprotein cholesterol (LDL-C) targets set by international guidelines.1 This is despite treatment with moderate to high intensity statins.1 This group of patients are at a high risk of CV events, and recurrent events are more likely to be fatal.1–4 For example, around 1 in 5 people who survive a myocardial infarction will have a recurrent event (like another MI, stroke or CV death) within 12 months.2 This event is two times more likely to be fatal compared to the first event.2
The good news is…lowering LDL-C levels is associated with fewer CV events5,6
Lipids have emerged in recent times as the most critical risk factor for cardiovascular (CV) events.7 Secondary prevention studies have shown that the lower the LDL-C the lower the risk of a CV event,5,6 with no threshold as yet identified where we fail to see a benefit.8 Professor Steve Nicholls said it best when he said, “No matter what the LDL-C level is, getting it lower is almost always better.”
|
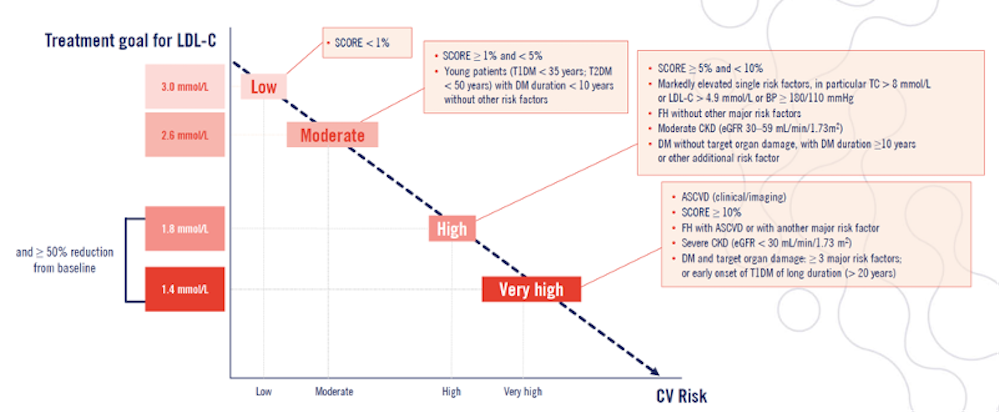
A note on risk As risk of a CV event increases, the LDL-C target decreases.9 Figure 1. 2019 ESC/EAS Dyslipidaemia guidelines categories of risk and their relation to risk of CV event occurring.9
ASCVD, atherosclerotic cardiovascular disease; CKD, chronic kidney disease; DM, diabetes mellitus; FH, familial hypercholesterolaemia; LDL-C, low-density lipoprotein cholesterol; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus. |
Getting to target and beyond with evolocumab (Repatha)
According to the 2019 ESC/EAS dyslipidaemia guideline treatment algorithm, lowering of LDL-C starts with lifestyle interventions before the addition of pharmacological therapies.9 These therapies are then added in a stepwise fashion, starting with highest tolerable statins, then adding ezetimibe and if the LDL-C is still not below the target adding in a PCSK9 inhibitor.9 This approach was developed due to the accumulative benefits of each therapy – as shown in Table 1.9
Table 1. Guideline-provided estimates of LDL-C lowering benefits of therapies.9
| Treatment | Average LDL-C reduction |
| Moderate-intensity statin | ~30% |
| High-intensity statin | ~50% |
| High-intensity statin + ezetimibe | ~65% |
| PCSK9 inhibitor (alone) | ~60% |
| PCSK9 inhibitor + high-intensity statin | ~75% |
| PCSK9 inhibitor + high-intensity statin + ezetimibe | ~85% |
Evolocumab (Repatha) is a human monoclonal antibody that binds selectively and with high affinity to PCSK9 in the blood and stops it from binding to LDL-C receptors on the liver cell surface. This prevents PCSK9-mediated degradation of these receptors resulting in associated reductions in serum LDL-C.11 The efficacy and safety of evolocumab (Repatha) was assessed in the Proficio clinical program – a suite of clinical trials conducted over almost a decade that looked at CV outcomes, consistency of LDL-C lowering, long-term safety and efficacy across various populations. The main study used in this webinar and associated case study webinars was the FOURIER trial, which looked at time to CV death, MI, hospitalisation for unstable angina, stroke or coronary revascularisation – whichever occurred first.12 Findings from this trial are outlined in Table 2.
Table 2: Repatha outcomes trial (FOURIER) findings12
| Results | |
| LDL-C levels | Reduced by 59% from baseline vs placebo |
| LDL-C <0.65 mmol/L | Achieved by 42% of patients (n=13,779) |
| Risk of CV death, MI, hospitalisation for unstable angina, stroke or coronary revascularisation | Reduced by 15% (reduced combined risk of CV death, MI and stroke by 20%) |
| Conclusions: | |
|
|
As noted by Professor Navar “an important note for patients is that the longer you are treated, the more benefit you get as patients who remained on treatment went from a 16% risk reduction after one year to a 25% risk reduction over the second and third years. Data like this can be a great motivator to help maintain compliance with treatment.” Professor Chow agreed and added “we are getting event reduction and no side effects! It’s nice to have the opportunity to do a bit more for these patients.”
Accessing evolocumab (Repatha) for high-risk patients just got easier: PBS reimbursement updates
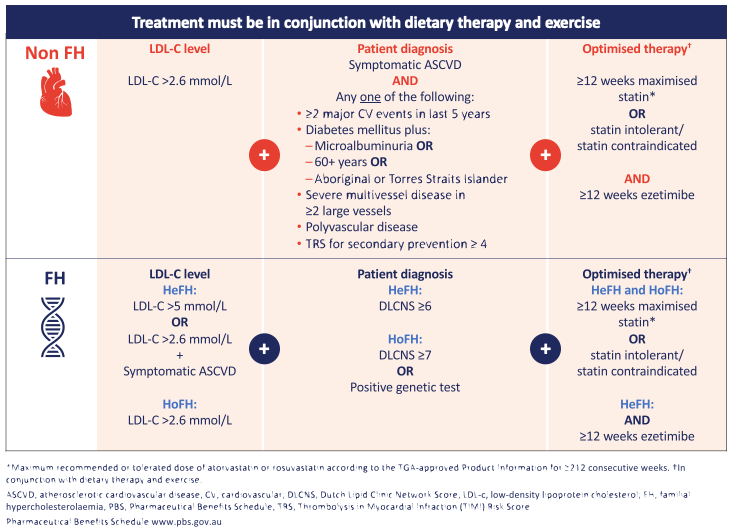
Professor Nicholls noted that previously in Australia, evolocumab (Repatha) was only listed on the PBS for patients with familial hypercholesterolaemia (FH). With the expanded eligibility criteria, shown in Figure 2, evolocumab (Repatha) can now be prescribed for patients with or without FH. Key changes to highlight include the addition of criteria for non-FH patients who have symptomatic ASCVD and at least one additional risk factor and, importantly that patients must have been optimally treated for more than 12 weeks with the maximum statin dose they can tolerate plus ezetimibe.13 Furthermore, the LDL-C qualifying level for FH was reduced from >3.3 mmol/L to >2.6 mmol/L.
Figure 2. Updated PBS criteria for evolocumab (Repatha).
Practical application of this update – identifying high-risk patients
Professor Navar was asked by a participant why it was so important to identify the highest risk patients? She answered “in every secondary analysis we’ve seen come out of FOURIER, people who are at higher predicted risk of events or who have higher observed event rates due to the presence of additional risk enhancers or other risk factors within the cardiovascular group are deriving more absolute benefit and though not statistically significant, in many cases an increased relative risk reduction in CV events as well. Although it’s approved for all patients with a previous CV event who need additional lipid lowering therapy beyond statins, the PBS criteria are designed to target those with established CVD who are at the highest risk of events and who may derive the highest absolute benefit.”
There are essentially five groups of patients who are considered high enough risk to access evolocumab (Repatha) reimbursement through the PBS. Identifying them and who they are were explored in more detail in the follow-up case study webinars, but best described by Professor Walter Abhayaratna of Canberra Health Services when he said, “I guess the first thing is, if you’ve got a person with coronary disease or prior stroke or established peripheral vascular disease, and you’ve tried your hardest to get their LDL cholesterol down to target, ask yourself the question ‘is this person suitable?’. So I start with that… The next thing to say is have they got enough of a high risk to be included, and that’s the middle column [referring to PBS eligibility criteria shown in Figure 2], so have they got multiple cardiovascular events, do they have complicated diabetes with a high risk, do they have multivessel disease, polyvascular disease or TIMI risk score for secondary prevention of ≥4, so they are the five groups that we refer to as high enough risk to be getting access to evolocumab (Repatha).”
Summary of practical advice from all presenters for managing patients on evolocumab (Repatha):
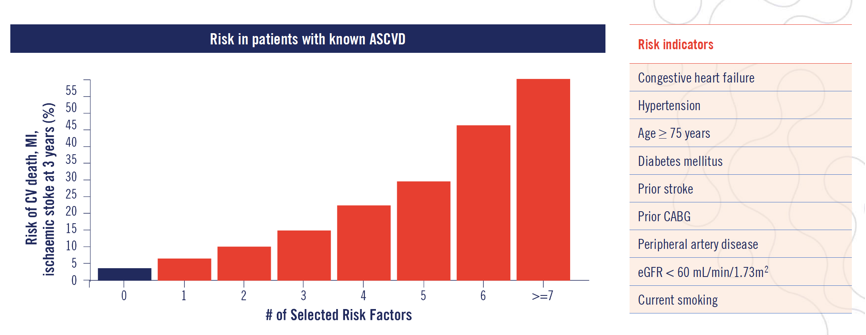
Figure 3. TIMI risk score for secondary prevention. For every risk indicator a patient has their risk of CV death, MI or ischaemic stroke within 3 years increases.14
|
References:
- Fox KM, et al. Clin Res Cardiol2018;107:380–88.
- Jernberg T, et al. Eur Heart J 2015;36:1163–70.
- Briffa TG, et al. Circ Cardiovasc Qual Outcomes 2011;4:107–13.
- Smolina K, et al. Circ Cardiovasc Qual Outcomes 2012;5:532–40.
- Cannon CP, et al. N EnglJ Med 2015;372:2387–97,
- LaRosa JC, et al. N EnglJ Med 2005;352:1425–35,
- Yusuf S, et al. Lancet.2004;364:937-52.
- Giugliano RP, et al. Lancet. 2017;390:1962–71.
- Mach F, et al. Eur Heart J 2020;41:111–188.
- Repatha (evolocumab) Approved Product Information.
- Sabatine MS, et al. N EngJ Med 2017;376:1713–22.
- Pharmaceutical Benefits Scheme www.pbs.gov.au.
- TIMI Study group TIMI Risk score for secondary prevention calculator www.timi.org.
- Bohula EA, et al. Circulation 2016;134:304–13.